Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients
- PMID: 33947344
- PMCID: PMC8093585
- DOI: 10.1186/s12879-021-06104-9
Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients
Abstract
Background and objectives: An effective treatment option is not yet available for SARS-CoV2, which causes the COVID-19 pandemic and whose effects are felt more and more every day. Ivermectin is among the drugs whose effectiveness in treatment has been investigated. In this study; it was aimed to investigate the presence of gene mutations that alter ivermectin metabolism and cause toxic effects in patients with severe COVID-19 pneumonia, and to evaluate the effectiveness and safety of ivermectin use in the treatment of patients without mutation.
Materials and methods: Patients with severe COVID19 pneumonia were included in the study, which was planned as a prospective, randomized, controlled, single-blind phase 3 study. Two groups, the study group and the control group, took part in the study. Ivermectin 200 mcg/kg/day for 5 days in the form of a solution prepared for enteral use added to the reference treatment protocol -hydroxychloroquine + favipiravir + azithromycin- of patients included in the study group. Patients in the control group were given only reference treatment with 3 other drugs without ivermectin. The presence of mutations was investigated by performing sequence analysis in the mdr1/abcab1 gene with the Sanger method in patients included in the study group according to randomization. Patients with mutations were excluded from the study and ivermectin treatment was not continued. Patients were followed for 5 days after treatment. At the end of the treatment and follow-up period, clinical response and changes in laboratory parameters were evaluated.
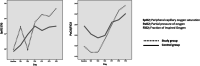
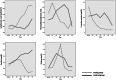
Results: A total of 66 patients, 36 in the study group and 30 in the control group were included in the study. Mutations affecting ivermectin metabolism was detected in genetic tests of six (16.7%) patients in the study group and they were excluded from the study. At the end of the 5-day follow-up period, the rate of clinical improvement was 73.3% (22/30) in the study group and was 53.3% (16/30) in the control group (p = 0.10). At the end of the study, mortality developed in 6 patients (20%) in the study group and in 9 (30%) patients in the control group (p = 0.37). At the end of the follow-up period, the average peripheral capillary oxygen saturation (SpO2) values of the study and control groups were found to be 93.5 and 93.0%, respectively. Partial pressure of oxygen (PaO2)/FiO2 ratios were determined as 236.3 ± 85.7 and 220.8 ± 127.3 in the study and control groups, respectively. While the blood lymphocyte count was higher in the study group compared to the control group (1698 ± 1438 and 1256 ± 710, respectively) at the end of the follow-up period (p = 0.24); reduction in serum C-reactive protein (CRP), ferritin and D-dimer levels was more pronounced in the study group (p = 0.02, p = 0.005 and p = 0.03, respectively).
Conclusions: According to the findings obtained, ivermectin can provide an increase in clinical recovery, improvement in prognostic laboratory parameters and a decrease in mortality rates even when used in patients with severe COVID-19. Consequently, ivermectin should be considered as an alternative drug that can be used in the treatment of COVID-19 disease or as an additional option to existing protocols.
Keywords: COVID-19; Ivermectin; SARS CoV-2; Treatment.
Conflict of interest statement
The authors declare that they have neither financial nor non-financial competing interests.
Figures
References
-
- WHO Coronavirus Disease (COVID-19) Dashboard available at https://COVID-19.who.int/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous