Excellent option for mass testing during the SARS-CoV-2 pandemic: painless self-collection and direct RT-qPCR
- PMID: 33947425
- PMCID: PMC8094981
- DOI: 10.1186/s12985-021-01567-3
Excellent option for mass testing during the SARS-CoV-2 pandemic: painless self-collection and direct RT-qPCR
Abstract
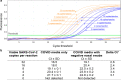
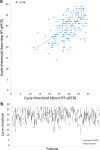
The early identification of asymptomatic yet infectious cases is vital to curb the 2019 coronavirus (COVID-19) pandemic and to control the disease in the post-pandemic era. In this paper, we propose a fast, inexpensive and high-throughput approach using painless nasal-swab self-collection followed by direct RT-qPCR for the sensitive PCR detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This approach was validated in a large prospective cohort study of 1038 subjects, analysed simultaneously using (1) nasopharyngeal swabs obtained with the assistance of healthcare personnel and analysed by classic two-step RT-qPCR on RNA isolates and (2) nasal swabs obtained by self-collection and analysed with direct RT-qPCR. Of these subjects, 28.6% tested positive for SARS-CoV-2 using nasopharyngeal swab sampling. Our direct RT-qPCR approach for self-collected nasal swabs performed well with results similar to those of the two-step RT-qPCR on RNA isolates, achieving 0.99 positive and 0.98 negative predictive values (cycle threshold [Ct] < 37). Our research also reports on grey-zone viraemia, including samples with near-cut-off Ct values (Ct ≥ 37). In all investigated subjects (n = 20) with grey-zone viraemia, the ultra-small viral load disappeared within hours or days with no symptoms. Overall, this study underscores the importance of painless nasal-swab self-collection and direct RT-qPCR for mass testing during the SARS-CoV-2 pandemic and in the post-pandemic era.
Keywords: COVID-19; Mass molecular testing; Nasal mid-turbinate swab; PCR diagnostics; Post-pandemic era; Self-collection.
Conflict of interest statement
E.K., R.F., M.R., P.Sa., J.M., M.D., O.J., P.St., M.K. have no conflict of interest, P.K. is member of the board of directors of a company Institute of Applied Biotechnologies a.s. The funders and the company had no role in the design, execution, interpretation, or writing of the study.
Figures
Similar articles
-
Diagnostic Performance of Self-Collected Saliva Versus Nasopharyngeal Swab for the Molecular Detection of SARS-CoV-2 in the Clinical Setting.Microbiol Spectr. 2021 Dec 22;9(3):e0046821. doi: 10.1128/Spectrum.00468-21. Epub 2021 Nov 3. Microbiol Spectr. 2021. PMID: 34730436 Free PMC article.
-
Polyester nasal swabs collected in a dry tube are a robust and inexpensive, minimal self-collection kit for SARS-CoV-2 testing.PLoS One. 2021 Apr 14;16(4):e0245423. doi: 10.1371/journal.pone.0245423. eCollection 2021. PLoS One. 2021. PMID: 33852576 Free PMC article.
-
Value of swab types and collection time on SARS-COV-2 detection using RT-PCR assay.J Virol Methods. 2020 Dec;286:113974. doi: 10.1016/j.jviromet.2020.113974. Epub 2020 Sep 16. J Virol Methods. 2020. PMID: 32949663 Free PMC article.
-
Relative sensitivity of anterior nares and nasopharyngeal swabs for initial detection of SARS-CoV-2 in ambulatory patients: Rapid review and meta-analysis.PLoS One. 2021 Jul 20;16(7):e0254559. doi: 10.1371/journal.pone.0254559. eCollection 2021. PLoS One. 2021. PMID: 34283845 Free PMC article. Review.
-
Detection profile of SARS-CoV-2 using RT-PCR in different types of clinical specimens: A systematic review and meta-analysis.J Med Virol. 2021 Feb;93(2):719-725. doi: 10.1002/jmv.26349. Epub 2020 Aug 2. J Med Virol. 2021. PMID: 32706393 Free PMC article.
Cited by
-
Clinical Evaluation of Nasopharyngeal, Oropharyngeal, Nasal Swabs, and Saliva for the Detection of SARS-CoV-2 by Direct RT-PCR.Diagnostics (Basel). 2022 Apr 27;12(5):1091. doi: 10.3390/diagnostics12051091. Diagnostics (Basel). 2022. PMID: 35626247 Free PMC article.
-
The Diagnostic Performance of Various Clinical Specimens for the Detection of COVID-19: A Meta-Analysis of RT-PCR Studies.Diagnostics (Basel). 2023 Sep 26;13(19):3057. doi: 10.3390/diagnostics13193057. Diagnostics (Basel). 2023. PMID: 37835801 Free PMC article. Review.
-
COVID-19 with high-sensitivity CRP associated with worse dynamic clinical parameters and outcomes.Front Med (Lausanne). 2024 Apr 22;11:1346646. doi: 10.3389/fmed.2024.1346646. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38711780 Free PMC article.
-
The relationship between COVID-19 viral load and disease severity: A systematic review.Immun Inflamm Dis. 2022 Mar;10(3):e580. doi: 10.1002/iid3.580. Epub 2021 Dec 13. Immun Inflamm Dis. 2022. PMID: 34904379 Free PMC article.
-
A systematic literature review on public health and healthcare resources for pandemic preparedness planning.BMC Public Health. 2024 Nov 11;24(1):3114. doi: 10.1186/s12889-024-20629-z. BMC Public Health. 2024. PMID: 39529010 Free PMC article.
References
-
- Al-Rifai RH, Acuna J, Al Hossany FI, Aden B, Al Memari SA, Al Mazrouei SK, Ahmed LA. Epidemiological characterization of symptomatic and asymptomatic COVID-19 cases and positivity in subsequent RT-PCR tests in the United Arab Emirates. PLoS ONE. 2021;16:e0246903. doi: 10.1371/journal.pone.0246903. - DOI - PMC - PubMed
-
- Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci AM, Salanti G, Low N. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med. 2020;17:e1003346. doi: 10.1371/journal.pmed.1003346. - DOI - PMC - PubMed
-
- Byambasuren O, Cardona M, Bell K, Clark J, McLaws M-L, Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. J Assoc Med Microbiol Infect Dis Canada. 2020;5:223–234. doi: 10.3138/jammi-2020-0030. - DOI - PMC - PubMed
-
- CDC, 2020. Interim guidelines for collecting, handling, and testing clinical specimens for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specim.... Accessed on 16 March 2021.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

