Recent advances in bioprinting technologies for engineering cardiac tissue
- PMID: 33947551
- PMCID: PMC9381603
- DOI: 10.1016/j.msec.2021.112057
Recent advances in bioprinting technologies for engineering cardiac tissue
Abstract
Annually increasing incidence of cardiac-related disorders and cardiac tissue's minimal regenerative capacity have motivated the researchers to explore effective therapeutic strategies. In the recent years, bioprinting technologies have witnessed a great wave of enthusiasm and have undergone steady advancements over a short period, opening the possibilities for recreating engineered functional cardiac tissue models for regenerative and diagnostic applications. With this perspective, the current review delineates recent developments in the sphere of engineered cardiac tissue fabrication, using traditional and advanced bioprinting strategies. The review also highlights different printing ink formulations, available cellular opportunities, and aspects of personalized medicines in the context of cardiac tissue engineering and bioprinting. On a concluding note, current challenges and prospects for further advancements are also discussed.
Keywords: Bioprinting; Cardiac tissue engineering; Cardio-inductive microenvironment; Stem cells.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement
All authors declare no conflict of interest either among themselves or with any funding agency.
Figures
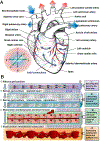
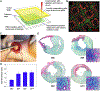
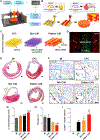

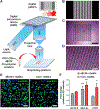
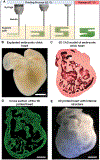
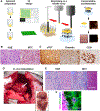

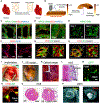
References
-
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association, Circulation 139 (2019) e56–e528. 10.1161/CIR.0000000000000659. - DOI - PubMed
-
- Wilcken DEL, Physiology of the normal heart, Medicine (Baltimore) 38 (2010) 336–339. 10.1016/j.mpmed.2010.03.014. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

