Altered β-Cell Prohormone Processing and Secretion in Type 1 Diabetes
- PMID: 33947721
- PMCID: PMC8173804
- DOI: 10.2337/dbi20-0034
Altered β-Cell Prohormone Processing and Secretion in Type 1 Diabetes
Abstract
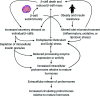
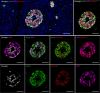
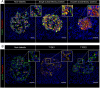
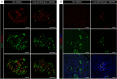
Analysis of data from clinical cohorts, and more recently from human pancreatic tissue, indicates that reduced prohormone processing is an early and persistent finding in type 1 diabetes. In this article, we review the current state of knowledge regarding alterations in islet prohormone expression and processing in type 1 diabetes and consider the clinical impact of these findings. Lingering questions, including pathologic etiologies and consequences of altered prohormone expression and secretion in type 1 diabetes, and the natural history of circulating prohormone production in health and disease, are considered. Finally, key next steps required to move forward in this area are outlined, including longitudinal testing of relevant clinical populations, studies that probe the genetics of altered prohormone processing, the need for combined functional and histologic testing of human pancreatic tissues, continued interrogation of the intersection between prohormone processing and autoimmunity, and optimal approaches for analysis. Successful resolution of these questions may offer the potential to use altered prohormone processing as a biomarker to inform therapeutic strategies aimed at personalized intervention during the natural history of type 1 diabetes and as a pathogenic anchor for identification of potential disease-specific endotypes.
© 2021 by the American Diabetes Association.
Figures

References
-
- Westermark P, Andersson A, Westermark GT.. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev 2011;91:795–826 - PubMed
-
- Weiss M, Steiner DF, Philipson LH.. Insulin biosynthesis, secretion, structure, and structure-activity relationships. In Endotext. Feingold KR, Anawalt B, Boyce A, et al., Eds. South Dartmouth, MA, 2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

