Ultrafast X-ray scattering offers a structural view of excited-state charge transfer
- PMID: 33947814
- PMCID: PMC8126834
- DOI: 10.1073/pnas.2021714118
Ultrafast X-ray scattering offers a structural view of excited-state charge transfer
Abstract
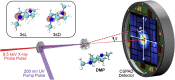
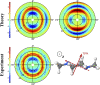
Intramolecular charge transfer and the associated changes in molecular structure in N,N'-dimethylpiperazine are tracked using femtosecond gas-phase X-ray scattering. The molecules are optically excited to the 3p state at 200 nm. Following rapid relaxation to the 3s state, distinct charge-localized and charge-delocalized species related by charge transfer are observed. The experiment determines the molecular structure of the two species, with the redistribution of electron density accounted for by a scattering correction factor. The initially dominant charge-localized state has a weakened carbon-carbon bond and reorients one methyl group compared with the ground state. Subsequent charge transfer to the charge-delocalized state elongates the carbon-carbon bond further, creating an extended 1.634 Å bond, and also reorients the second methyl group. At the same time, the bond lengths between the nitrogen and the ring-carbon atoms contract from an average of 1.505 to 1.465 Å. The experiment determines the overall charge transfer time constant for approaching the equilibrium between charge-localized and charge-delocalized species to 3.0 ps.
Keywords: X-ray scattering; charge transfer; excited state; femtosecond; ultrafast dynamics.
Conflict of interest statement
The authors declare no competing interest.
Figures






References
-
- Zewail A. H., Femtochemistry: Atomic-scale dynamics of the chemical bond using ultrafast lasers (Nobel lecture). Angew. Chem. Int. Ed. Engl. 39, 2586–2631 (2000). - PubMed
-
- Frischmann P. D., Mahata K., Würthner F., Powering the future of molecular artificial photosynthesis with light-harvesting metallosupramolecular dye assemblies. Chem. Soc. Rev. 42, 1847–1870 (2013). - PubMed
-
- Kuleff A. I., Cederbaum L. S., Charge migration in different conformers of glycine: The role of nuclear geometry. Chem. Phys. 338, 320–328 (2007).
-
- Lünnemann S., Kuleff A. I., Cederbaum L. S., Charge migration following ionization in systems with chromophore-donor and amine-acceptor sites. J. Chem. Phys. 129, 104305 (2008). - PubMed

