A hypothalamic-thalamostriatal circuit that controls approach-avoidance conflict in rats
- PMID: 33947849
- PMCID: PMC8097010
- DOI: 10.1038/s41467-021-22730-y
A hypothalamic-thalamostriatal circuit that controls approach-avoidance conflict in rats
Abstract
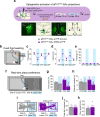
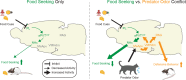
Survival depends on a balance between seeking rewards and avoiding potential threats, but the neural circuits that regulate this motivational conflict remain largely unknown. Using an approach-food vs. avoid-predator threat conflict test in rats, we identified a subpopulation of neurons in the anterior portion of the paraventricular thalamic nucleus (aPVT) which express corticotrophin-releasing factor (CRF) and are preferentially recruited during conflict. Inactivation of aPVTCRF neurons during conflict biases animal's response toward food, whereas activation of these cells recapitulates the food-seeking suppression observed during conflict. aPVTCRF neurons project densely to the nucleus accumbens (NAc), and activity in this pathway reduces food seeking and increases avoidance. In addition, we identified the ventromedial hypothalamus (VMH) as a critical input to aPVTCRF neurons, and demonstrated that VMH-aPVT neurons mediate defensive behaviors exclusively during conflict. Together, our findings describe a hypothalamic-thalamostriatal circuit that suppresses reward-seeking behavior under the competing demands of avoiding threats.
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- Lima SL, Dill LM. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 1989;68:619–640. doi: 10.1139/z90-092. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

