Gastrokine-1, an anti-amyloidogenic protein secreted by the stomach, regulates diet-induced obesity
- PMID: 33947892
- PMCID: PMC8096951
- DOI: 10.1038/s41598-021-88928-8
Gastrokine-1, an anti-amyloidogenic protein secreted by the stomach, regulates diet-induced obesity
Abstract
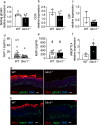
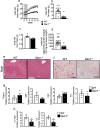
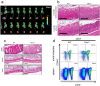
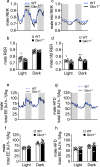
Obesity and its sequelae have a major impact on human health. The stomach contributes to obesity in ways that extend beyond its role in digestion, including through effects on the microbiome. Gastrokine-1 (GKN1) is an anti-amyloidogenic protein abundantly and specifically secreted into the stomach lumen. We examined whether GKN1 plays a role in the development of obesity and regulation of the gut microbiome. Gkn1-/- mice were resistant to diet-induced obesity and hepatic steatosis (high fat diet (HFD) fat mass (g) = 10.4 ± 3.0 (WT) versus 2.9 ± 2.3 (Gkn1-/-) p < 0.005; HFD liver mass (g) = 1.3 ± 0.11 (WT) versus 1.1 ± 0.07 (Gkn1-/-) p < 0.05). Gkn1-/- mice also exhibited increased expression of the lipid-regulating hormone ANGPTL4 in the small bowel. The microbiome of Gkn1-/- mice exhibited reduced populations of microbes implicated in obesity, namely Firmicutes of the class Erysipelotrichia. Altered metabolism consistent with use of fat as an energy source was evident in Gkn1-/- mice during the sleep period. GKN1 may contribute to the effects of the stomach on the microbiome and obesity. Inhibition of GKN1 may be a means to prevent obesity.
Conflict of interest statement
DLB owns Lumen Bio LLC, which has patents filed related to gastrokine-1 and obesity. DLB receives research support from Pfizer. R.J.S. receives research support from Ethicon Endo-Surgery/Johnson & Johnson, Novo Nordisk, Janssen/Johnson & Johnson, Medimmune, MedImmune, Kalyope and Sanofi. R.J.S. serves as a consultant for Ethicon Endo-Surgery/Johnson & Johnson, Novartis, Orexigen, Novo Nordisk, Daiichi Sankyo, Janssen/Johnson & Johnson, Paul Hastings Law Firm, Kallyope and Scohia. All other authors declare no conflicts.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

