The Stability of Dentin Surface Biobarrier Consisting of Mesoporous Delivery System on Dentinal Tubule Occlusion and Streptococcus Mutans Biofilm Inhibition
- PMID: 33948084
- PMCID: PMC8088303
- DOI: 10.2147/IJN.S290254
The Stability of Dentin Surface Biobarrier Consisting of Mesoporous Delivery System on Dentinal Tubule Occlusion and Streptococcus Mutans Biofilm Inhibition
Abstract
Background: The dentin exposure always leads to dentin hypersensitivity and/or caries. Given the dentin's tubular structure and low mineralization degree, reestablishing an effective biobarrier to stably protect dentin remains significantly challenging. This study reports a versatile dentin surface biobarrier consisting of a mesoporous silica-based epigallocatechin-3-gallate (EGCG)/nanohydroxyapatite delivery system and evaluates its stability on the dentinal tubule occlusion and the Streptococcus mutans (S. mutans) biofilm inhibition.
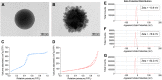
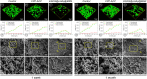
Materials and methods: The mesoporous delivery system was fabricated and characterized. Sensitive dentin discs were prepared and randomly allocated to three groups: 1, control group; 2, casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) group; and 3, the mesoporous delivery system group. The dentin permeability, dentinal tubule occlusion, acid and abrasion resistance, and S. mutans biofilm inhibition were determined for 1 week and 1 month. The in vitro release profiles of EGCG, Ca, and P were also monitored.
Results: The mesoporous delivery system held the ability to sustainably release EGCG, Ca, and P and could persistently occlude dentinal tubules with acid and abrasion resistance, reduce the dentin permeability, and inhibit the S. mutans biofilm formation for up to 1 month compared with the two other groups. The system provided prolonged stability to combat oral adverse challenges and served as an effective surface biobarrier to protect the exposed dentin.
Conclusion: The establishment of the dentin surface biobarrier consisting of a mesoporous delivery system indicates a promising strategy for the prevention and the management of dentin hypersensitivity and caries after enamel loss.
Keywords: biofilm; dentin; epigallocatechin-3-gallate; mesoporous silica; nanohydroxyapatite.
© 2021 Yu et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures








References
-
- Lawn BR, Lee JJW, Chai H. Teeth: among nature’s most durable biocomposites. Annu Rev Mat Res. 2010;40(1):55–75. doi:10.1146/annurev-matsci-070909-104537 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

