Inflammation in multiple sclerosis
- PMID: 33948118
- PMCID: PMC8053832
- DOI: 10.1177/17562864211007687
Inflammation in multiple sclerosis
Abstract
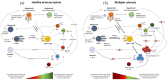
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) that is characterised pathologically by demyelination, gliosis, neuro-axonal damage and inflammation. Despite intense research, the underlying pathomechanisms driving inflammatory demyelination in MS still remain incompletely understood. It is thought to be caused by an autoimmune response towards CNS self-antigens in genetically susceptible individuals, assuming autoreactive T cells as disease-initiating immune cells. Yet, B cells were recognized as crucial immune cells in disease pathology, including antibody-dependent and independent effects. Moreover, myeloid cells are important contributors to MS pathology, and it is becoming increasingly evident that different cell types act in concert during MS immunopathology. This is supported by the finding that the beneficial effects of actual existing disease-modifying therapies cannot be attributed to one single immune cell-type, but rather involve immunological cooperation. The current strategy of MS therapies thus aims to shift the immune cell repertoire from a pro-inflammatory towards an anti-inflammatory phenotype, involving regulatory T and B cells and anti-inflammatory macrophages. Although no existing therapy actually exists that directly induces an enhanced regulatory immune cell pool, numerous studies identified potential net effects on these cell types. This review gives a conceptual overview on T cells, B cells and myeloid cells in the immunopathology of relapsing-remitting MS and discusses potential contributions of actual disease-modifying therapies on these immune cell phenotypes.
Keywords: B cells; T cells; immune network; immune regulation; inflammation; myeloid cells; relapsing-remitting multiple sclerosis.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures
References
-
- Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain 2006; 129: 1953–1971. - PubMed
-
- Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372: 1502–1517. - PubMed
-
- Genain CP, Cannella B, Hauser SL, et al. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med 1999; 5: 170–175. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

