Micro-scale technologies propel biology and medicine
- PMID: 33948133
- PMCID: PMC8081554
- DOI: 10.1063/5.0047196
Micro-scale technologies propel biology and medicine
Abstract
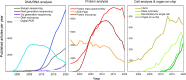
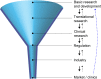
Historically, technology has been central to new discoveries in biology and progress in medicine. Among various technologies, microtechnologies, in particular, have had a prominent role in the revolution experienced by the life sciences in the last few decades, which will surely continue in the years to come. In this Perspective, we illustrate how microtechnologies, with a focus on microfluidics, have evolved in trends/waves to tackle the boundary of knowledge in the life sciences. We provide illustrative examples of technology-enabled biological breakthroughs and their current and future use in clinics. Finally, we take a closer look at the translational process to understand why the incorporation of new micro-scale technologies in medicine has been comparatively slow so far.
© 2021 Author(s).
Figures



Similar articles
-
Twenty five years of the National Academy of Medical Sciences of Ukraine - progress and priorities for future of radiation medicine and biology.Probl Radiac Med Radiobiol. 2017 Dec;22:10-14. Probl Radiac Med Radiobiol. 2017. PMID: 29286493 English, Ukrainian.
-
Physical Biology : challenges for our second decade.Phys Biol. 2014 Jun;11(3):030201. doi: 10.1088/1478-3975/11/3/030201. Epub 2014 Apr 15. Phys Biol. 2014. PMID: 24732666
-
Divide and conquer: A perspective on biochips for single-cell and rare-molecule analysis by next-generation sequencing.APL Bioeng. 2019 Jun 25;3(2):020901. doi: 10.1063/1.5095962. eCollection 2019 Jun. APL Bioeng. 2019. PMID: 31431936 Free PMC article.
-
Emerging Trends in Micro- and Nanoscale Technologies in Medicine: From Basic Discoveries to Translation.ACS Nano. 2017 Jun 27;11(6):5195-5214. doi: 10.1021/acsnano.7b01493. Epub 2017 May 19. ACS Nano. 2017. PMID: 28524668 Review.
-
Next-Generation Sequencing: The Translational Medicine Approach from "Bench to Bedside to Population".Medicines (Basel). 2016 Jun 2;3(2):14. doi: 10.3390/medicines3020014. Medicines (Basel). 2016. PMID: 28930123 Free PMC article. Review.
Cited by
-
Tumor-on-chip platforms for breast cancer continuum concept modeling.Front Bioeng Biotechnol. 2024 Oct 2;12:1436393. doi: 10.3389/fbioe.2024.1436393. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39416279 Free PMC article. Review.
-
Enhancing the Efficiency of Conventional Surface Immunoassays Within Standard Labware Using Microscale Flows.Methods Mol Biol. 2024;2804:103-115. doi: 10.1007/978-1-0716-3850-7_6. Methods Mol Biol. 2024. PMID: 38753143
References
-
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G. F., and Tan W., “A novel coronavirus from patients with pneumonia in China, 2019,” N. Engl. J. Med. 382(8), 727–733 (2020). 10.1056/NEJMoa2001017 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
