EZH2 is involved in psoriasis progression by impairing miR-125a-5p inhibition of SFMBT1 and leading to inhibition of the TGFβ/SMAD pathway
- PMID: 33948156
- PMCID: PMC8053822
- DOI: 10.1177/2040622320987348
EZH2 is involved in psoriasis progression by impairing miR-125a-5p inhibition of SFMBT1 and leading to inhibition of the TGFβ/SMAD pathway
Abstract
Aims: In this study, we aimed to decipher the impact of enhancer of zeste homolog 2 (EZH2) in psoriasis as well as the underlying mechanism.
Methods: A mouse model of psoriasis was developed by means of imiquimod induction, with the expression of EZH2, microRNA-125a-5p (miR-125a-5p), and SFMBT1 determined. The role of EZH2, miR-125a-5p, and SFMBT1 in malignant phenotypes of HaCaT cells and the development of psoriasis in vivo was subsequently investigated through gain- and loss-of-function experiments. Chromatin immunoprecipitation assay and dual-luciferase reporter assay were conducted to explore the relationship between EZH2 or SFMBT1 and miR-125a-5p. Finally, the effects of EZH2 and miR-125a-5p on the transforming growth factor β (TGFβ)/SMAD pathway were analyzed.
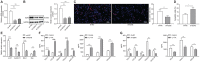
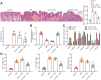
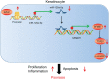
Results: Overexpressed SFMBT1 and EZH2 was detected while miR-125a-5p were downregulated in psoriasis tissues and human keratinocyte (HaCaT) cells. EZH2 increased the levels of IL-17A-induced cytokines and promoted the malignant phenotypes of HaCaT cells. Functionally, EZH2 reduced miR-125a-5p expression while miR-125a-5p targeted SFMBT1 to activate the TGFβ/SMAD pathway in vitro. Knockdown of EZH2 or up-regulation of miR-125a-5p inhibited cell proliferation and the levels of IL-17A-induced cytokines, but increased the expression of TGFβ1 and the extent of smad2 and smad3 phosphorylation in HaCaT cells. Notably, EZH2 contributed to the development of psoriasis in vivo by inhibiting the TGFβ/SMAD pathway via impairment of miR-125a-5p-mediated SFMBT1 inhibition.
Conclusion: Taken together, the results of the current study highlight the ability of EZH2 to potentially inactivate the TGFβ/SMAD pathway via upregulation of miR-125a-5p-dependent SFMBT1during the progression of psoriatic lesions.
Keywords: EZH2; SFMBT1; TGFβ/SMAD pathway; microRNA-125a-5p; psoriasis.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures




Similar articles
-
MiR-20a-3p regulates TGF-β1/Survivin pathway to affect keratinocytes proliferation and apoptosis by targeting SFMBT1 in vitro.Cell Signal. 2018 Sep;49:95-104. doi: 10.1016/j.cellsig.2018.06.003. Epub 2018 Jun 7. Cell Signal. 2018. PMID: 29886071
-
MicroRNA-125a-5p regulates the effect of Tregs on Th1 and Th17 through targeting ETS-1/STAT3 in psoriasis.J Transl Med. 2023 Sep 29;21(1):678. doi: 10.1186/s12967-023-04427-6. J Transl Med. 2023. PMID: 37773129 Free PMC article.
-
MicroRNA-34b-5p binds enhancer of zeste 2 to inhibit milk fat globule-EGF factor 8 expression, affecting liver fibrosis.J Physiol Biochem. 2022 Nov;78(4):885-895. doi: 10.1007/s13105-022-00914-4. Epub 2022 Sep 23. J Physiol Biochem. 2022. PMID: 36138295
-
MicroRNA-125a Correlates with Decreased Psoriasis Severity and Inflammation and Represses Keratinocyte Proliferation.Dermatology. 2021;237(4):568-578. doi: 10.1159/000510681. Epub 2021 Mar 18. Dermatology. 2021. PMID: 33735868
-
MiR-125a-5p ameliorates monocrotaline-induced pulmonary arterial hypertension by targeting the TGF-β1 and IL-6/STAT3 signaling pathways.Exp Mol Med. 2018 Apr 27;50(4):1-11. doi: 10.1038/s12276-018-0068-3. Exp Mol Med. 2018. PMID: 29700287 Free PMC article.
Cited by
-
Emerging roles of non-coding RNAs in psoriasis pathogenesis.Funct Integr Genomics. 2023 Apr 19;23(2):129. doi: 10.1007/s10142-023-01057-5. Funct Integr Genomics. 2023. PMID: 37072609 Review.
-
Multi-Omics Approach to Improved Diagnosis and Treatment of Atopic Dermatitis and Psoriasis.Int J Mol Sci. 2024 Jan 15;25(2):1042. doi: 10.3390/ijms25021042. Int J Mol Sci. 2024. PMID: 38256115 Free PMC article. Review.
-
Mouse Models of Psoriasis: A Comprehensive Review.J Invest Dermatol. 2022 Mar;142(3 Pt B):884-897. doi: 10.1016/j.jid.2021.06.019. Epub 2021 Dec 23. J Invest Dermatol. 2022. PMID: 34953514 Free PMC article. Review.
-
Gene set enrichment analysis and ingenuity pathway analysis to verify the impact of Wnt signaling in psoriasis treated with Taodan granules.Am J Transl Res. 2023 Jan 15;15(1):422-434. eCollection 2023. Am J Transl Res. 2023. PMID: 36777818 Free PMC article.
-
Unveiling the Role of Histone Methyltransferases in Psoriasis Pathogenesis: Insights from Transcriptomic Analysis.Int J Mol Sci. 2025 Jun 30;26(13):6329. doi: 10.3390/ijms26136329. Int J Mol Sci. 2025. PMID: 40650108 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

