Enhancing reproducibility using interprofessional team best practices
- PMID: 33948243
- PMCID: PMC8057443
- DOI: 10.1017/cts.2020.512
Enhancing reproducibility using interprofessional team best practices
Abstract
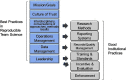
The pervasive problem of irreproducibility of preclinical research represents a substantial threat to the translation of CTSA-generated health interventions. Key stakeholders in the research process have proposed solutions to this challenge to encourage research practices that improve reproducibility. However, these proposals have had minimal impact, because they either 1. take place too late in the research process, 2. focus exclusively on the products of research instead of the processes of research, and/or 3. fail to take into account the driving incentives in the research enterprise. Because so much clinical and translational science is team-based, CTSA hubs have a unique opportunity to leverage Science of Team Science research to implement and support innovative, evidence-based, team-focused, reproducibility-enhancing activities at a project's start, and across its evolution. Here, we describe the impact of irreproducibility on clinical and translational science, review its origins, and then describe stakeholders' efforts to impact reproducibility, and why those efforts may not have the desired effect. Based on team-science best practices and principles of scientific integrity, we then propose ways for Translational Teams to build reproducible behaviors. We end with suggestions for how CTSAs can leverage team-based best practices and identify observable behaviors that indicate a culture of reproducible research.
Keywords: Team science; ethics; interprofessional teams; reproducibility; translational teams.
© The Association for Clinical and Translational Science 2020.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- Begley CG, Ioannidis JP. Reproducibility in science: improving the standard for basic and preclinical research. Circulation Research 2015; 116(1) :116–126. - PubMed
-
- Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nature Reviews Drug Discovery 2011; 10(9): 712. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
