125I seed implantation brachytherapy for glottic carcinoma: an experimental and clinical study
- PMID: 33948360
- PMCID: PMC8085880
125I seed implantation brachytherapy for glottic carcinoma: an experimental and clinical study
Abstract
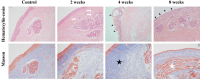
125I seed implantation brachytherapy (ISIB) is the preferred treatment for prostate cancer. Is ISIB technically suitable for glottic carcinoma (GC)? This question has not been answered in the literature; thus, the present study was carried out to evaluate the feasibility and effect of ISIB on GC in animal and clinical studies. An animal model of Tu-212 cell laryngeal carcinoma xenografts (n = 20 animals) underwent ISIB treatments [experimental group (EG) using 0.8-mCi/seed, control group (CG) using 0-mCi/seed]; at 4 weeks, haematoxylin-eosin (HE) staining was performed, and the mRNA and protein expression of Bax, Bcl-2 and PCNA was analysed. Moreover, thirty healthy beagle dogs underwent ISIB under CT guidance (EG, 0.8 mCi/seed, CG, 0 mCi/seed), and injuries to the normal tissue were analysed by HE and Masson staining at 2, 4, and 8 weeks. Finally, twenty-one GC patients (T2-3N0M0) underwent percutaneous ISIB at a mean prescription dose of 116.8 Gy; the technical success, complications, local tumour response, voice quality, local progression and overall survival were analysed. The results showed that the xenograft tumours were significantly inhibited in the EG. The Bax protein levels were significantly increased in this group (P<0.05), while the Bcl-2 and PCNA protein levels were decreased (P<0.05). Moreover, the glottic injury scores increased with the dose accumulation (P<0.05), while the adjacent tissue did not show pathohistological injury, and the routine blood tests showed no change between the pre-treatment baseline levels and the levels 2, 4, or 8 weeks later (P>0.05). The clinical study found that the rate of technical success was 100% with no procedure-related complications; furthermore, complete response was achieved in all patients, and no local progression occurred. All patients survived and showed improvements in their voice quality (P<0.05) during the follow-up period (median 23.5 months). The results show that ISIB is a safe and effective treatment for GC; randomized controlled trials are needed to further evaluate its clinical efficacy.
Keywords: 125I brachytherapy; clinical study; experimental study; glottic carcinoma.
AJCR Copyright © 2021.
Conflict of interest statement
None.
Figures







Similar articles
-
Percutaneous computed tomography/ultrasonography-guided permanent iodine-125 implantation as salvage therapy for recurrent squamous cell cancers of head and neck.Cancer Biol Ther. 2010 Jun 15;9(12):959-66. doi: 10.4161/cbt.9.12.11700. Cancer Biol Ther. 2010. PMID: 20873398 Clinical Trial.
-
Clinical effectiveness of 125I-seed implantation in combination with nimotuzumab therapy for the advanced oral carcinoma: preliminary results.Eur Rev Med Pharmacol Sci. 2014;18(21):3304-10. Eur Rev Med Pharmacol Sci. 2014. PMID: 25487943 Clinical Trial.
-
Permanent interstitial 125I seed implantation as a salvage therapy for pediatric recurrent or metastatic soft tissue sarcoma after multidisciplinary treatment.World J Surg Oncol. 2015 Dec 15;13:335. doi: 10.1186/s12957-015-0747-7. World J Surg Oncol. 2015. PMID: 26666635 Free PMC article.
-
Effect of radiation fraction size on local control rates for early glottic carcinoma. A model analysis for in vivo tumor growth and radio-response parameters.Arch Otolaryngol Head Neck Surg. 1994 Jul;120(7):737-42. doi: 10.1001/archotol.1994.01880310041008. Arch Otolaryngol Head Neck Surg. 1994. PMID: 8018326 Review.
-
Does brachytherapy have a role in the treatment of prostate cancer?Hematol Oncol Clin North Am. 1996 Jun;10(3):653-73. doi: 10.1016/s0889-8588(05)70359-2. Hematol Oncol Clin North Am. 1996. PMID: 8773503 Review.
Cited by
-
Personalized 125I Seed Interstitial Brachytherapy for Patients Aged 80 Years and Over with Early Primary High-risk Non-melanoma Skin Cancer.Curr Radiopharm. 2024;17(2):163-167. doi: 10.2174/0118744710240256231103095018. Curr Radiopharm. 2024. PMID: 38018208
-
Identification of a novel peptide targeting TIGIT to evaluate immunomodulation of 125I seed brachytherapy in HCC by near-infrared fluorescence.Front Oncol. 2023 Apr 14;13:1143266. doi: 10.3389/fonc.2023.1143266. eCollection 2023. Front Oncol. 2023. PMID: 37124530 Free PMC article.
References
-
- Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, Peters G, Lee DJ, Leaf A, Ensley J, Cooper J. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. - PubMed
-
- Wolber P, Schwarz D, Stange T, Ortmann M, Balk M, Anagiotos A, Gostian AO. Surgical treatment for early stage glottic carcinoma with involvement of the anterior commissure. Otolaryngol Head Neck Surg. 2018;158:295–302. - PubMed
-
- Succo G, Fantini M, Rizzotto G. Supratracheal partial laryngectomy: indications, oncologic and functional results. Curr Opin Otolaryngol Head Neck Surg. 2017;25:127–132. - PubMed
-
- Saraniti C, Speciale R, Santangelo M, Massaro N, Maniaci A, Gallina S, Serra A, Cocuzza S. Functional outcomes after supracricoid modified partial laryngectomy. J Biol Regul Homeost Agents. 2019;33:1903–1907. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
