Updated guidance on the management of cancer treatment-induced bone loss (CTIBL) in pre- and postmenopausal women with early-stage breast cancer
- PMID: 33948427
- PMCID: PMC8080519
- DOI: 10.1016/j.jbo.2021.100355
Updated guidance on the management of cancer treatment-induced bone loss (CTIBL) in pre- and postmenopausal women with early-stage breast cancer
Abstract
Introduction: Adjuvant endocrine therapy induces bone loss and increases fracture risk in women with hormone-receptor positive, early-stage breast cancer (EBC). We aimed to update a previous position statement on the management of aromatase inhibitors (AIs) induced bone loss and now included premenopausal women.
Methods: We conducted a systematic literature search of the medical databases from January 2017 to May 2020 and assessed 144 new studies.
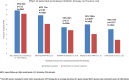
Results: Extended use of AIs beyond 5 years leads to persistent bone loss in breast cancer extended adjuvant trials and meta-analyses. In addition to bone mineral density (BMD), vertebral fracture assessment (VFA) and trabecular bone score (TBS) were shown to independently predict fracture risk in real life prospective studies. FRAX® tool does not seem to be reliable for assessing fracture risk in CTIBL. In premenopausal women, there is strong evidence that intravenous zoledronate prevents bone loss but weak conflicting evidence on reducing disease recurrence from independent randomised controlled trials (RCTs). In postmenopausal women, the strongest evidence for fracture prevention is for denosumab based on a well-powered RCT while there is strong evidence for bisphosphonates (BPs) to prevent and reduce CTIBL but no convincing data on fractures. Adjuvant denosumab has failed to show anticancer benefits in a large, well-designed RCT.
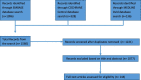
Discussion and conclusions: Extended use of AIs and persistent bone loss from recent data reinforce the need to evaluate fracture risk in EBC women initiated on AIs. Fracture risk should be assessed with clinical risk factors and BMD along with VFA, but FRAX is not adapted to CTIBL. Anti-resorptive therapy should be considered in those with a BMD T-score < -2.0 SD or with ≥ 2 clinical risk factors including a BMD T-score < -1.0 SD. In premenopausal women, intravenous zoledronate is the only drug reported to prevent bone loss and may have additional anticancer benefits. In postmenopausal women, either denosumab or BPs can be prescribed for fracture prevention with pertinent attention to the rebound phenomenon after stopping denosumab. Adjuvant BPs, in contrast to denosumab, have shown high level evidence for reducing breast cancer recurrence in high-risk post-MP women which should be taken into account when choosing between these two.
Keywords: Aromatase inhibitors; Bisphosphonates; Bone loss; CABS, Cancer and Bone Society; Denosumab; Disease free survival; ECTS, European Calcified Tissue Society; ESCEO: European Society for Clinical and Economics Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases; Early-stage breast cancer; Fracture; IEG, International Expert Group for AIBL; IMS, International Menopause Society; IOF, International Osteoporosis Foundation; SIOG, International Society for Geriatric Oncology.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Winters S., Martin C., Murphy D., Shokar N.K. Breast Cancer Epidemiology, Prevention, and Screening. Prog. Mol. Biol. Transl. Sci. 2017;151:1–32. - PubMed
-
- Pierce S.M., Recht A., Lingos T.I., Abner A., Vicini F., Silver B. Long-term radiation complications following conservative surgery (CS) and radiation therapy (RT) in patients with early stage breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 1992;23(5):915–923. - PubMed
-
- van Leeuwen B.L., Hartel R.M., Jansen H.W., Kamps W.A., Hoekstra H.J. The effect of chemotherapy on the morphology of the growth plate and metaphysis of the growing skeleton. Eur. J. Surg. Oncol. 2003;29(1):49–58. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

