Early satellite cell communication creates a permissive environment for long-term muscle growth
- PMID: 33948557
- PMCID: PMC8080523
- DOI: 10.1016/j.isci.2021.102372
Early satellite cell communication creates a permissive environment for long-term muscle growth
Abstract
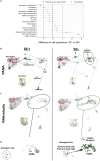
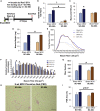
Using in vivo muscle stem cell (satellite cell)-specific extracellular vesicle (EV) tracking, satellite cell depletion, in vitro cell culture, and single-cell RNA sequencing, we show satellite cells communicate with other cells in skeletal muscle during mechanical overload. Early satellite cell EV communication primes the muscle milieu for proper long-term extracellular matrix (ECM) deposition and is sufficient to support sustained hypertrophy in adult mice, even in the absence of fusion to muscle fibers. Satellite cells modulate chemokine gene expression across cell types within the first few days of loading, and EV delivery of miR-206 to fibrogenic cells represses Wisp1 expression required for appropriate ECM remodeling. Late-stage communication from myogenic cells during loading is widespread but may be targeted toward endothelial cells. Satellite cells coordinate adaptation by influencing the phenotype of recipient cells, which extends our understanding of their role in muscle adaptation beyond regeneration and myonuclear donation.
Keywords: Cell Biology; Functional Aspects of Cell Biology.
© 2021 The Authors.
Conflict of interest statement
The authors have no conflicts to declare.
Figures






References
-
- Bergen V., Lange M., Peidli S., Wolf F.A., Theis F.J. Generalizing RNA velocity to transient cell states through dynamical modeling. Nat. Biotech. 2020;38:1408–1414. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

