Activated CD4+ T cells and CD14hiCD16+ monocytes correlate with antibody response following influenza virus infection in humans
- PMID: 33948570
- PMCID: PMC8080109
- DOI: 10.1016/j.xcrm.2021.100237
Activated CD4+ T cells and CD14hiCD16+ monocytes correlate with antibody response following influenza virus infection in humans
Abstract
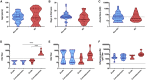
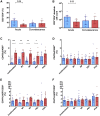
The failure to mount an antibody response following viral infection or seroconversion failure is a largely underappreciated and poorly understood phenomenon. Here, we identified immunologic markers associated with robust antibody responses after influenza virus infection in two independent human cohorts, SHIVERS and FLU09, based in Auckland, New Zealand and Memphis, Tennessee, USA, respectively. In the SHIVERS cohort, seroconversion significantly associates with (1) hospitalization, (2) greater numbers of proliferating, activated CD4+ T cells, but not CD8+ T cells, in the periphery during the acute phase of illness, and (3) fewer inflammatory monocytes (CD14hiCD16+) by convalescence. In the FLU09 cohort, fewer CD14hiCD16+ monocytes during early illness in the nasal mucosa were also associated with the generation of influenza-specific mucosal immunoglobulin A (IgA) and IgG antibodies. Our study demonstrates that seroconversion failure after infection is a definable immunological phenomenon, associated with quantifiable cellular markers that can be used to improve diagnostics, vaccine efficacy, and epidemiologic efforts.
Keywords: antibody; cellular immunity; humoral immunity; immune correlate; infection; influenza; monocytes; mucosal immunity; respiratory virus; seroconversion.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Chen M.I., Lee V.J., Lim W.Y., Barr I.G., Lin R.T., Koh G.C., Yap J., Cui L., Cook A.R., Laurie K. 2009 influenza A(H1N1) seroconversion rates and risk factors among distinct adult cohorts in Singapore. JAMA. 2010;303:1383–1391. - PubMed
-
- Huang K.Y., Li C.K., Clutterbuck E., Chui C., Wilkinson T., Gilbert A., Oxford J., Lambkin-Williams R., Lin T.Y., McMichael A.J., Xu X.N. Virus-specific antibody secreting cell, memory B-cell, and sero-antibody responses in the human influenza challenge model. J. Infect. Dis. 2014;209:1354–1361. - PubMed
-
- Memoli M.J., Shaw P.A., Han A., Czajkowski L., Reed S., Athota R., Bristol T., Fargis S., Risos K., Powers J.H. Evaluation of Antihemagglutinin and Antineuraminidase Antibodies as Correlates of Protection in an Influenza A/H1N1 Virus Healthy Human Challenge Model. MBio. 2016;7:e00417-16. - PMC - PubMed
-
- Murphy B.R., Rennels M.B., Douglas R.G., Jr., Betts R.F., Couch R.B., Cate T.R., Jr., Chanock R.M., Kendal A.P., Maassab H.F., Suwanagool S. Evaluation of influenza A/Hong Kong/123/77 (H1N1) ts-1A2 and cold-adapted recombinant viruses in seronegative adult volunteers. Infect. Immun. 1980;29:348–355. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

