IGFBP-2 partly mediates the early metabolic improvements caused by bariatric surgery
- PMID: 33948578
- PMCID: PMC8080239
- DOI: 10.1016/j.xcrm.2021.100248
IGFBP-2 partly mediates the early metabolic improvements caused by bariatric surgery
Abstract
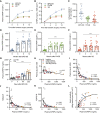
Insulin-like growth factor-binding protein (IGFBP)-2 is a circulating biomarker of cardiometabolic health. Here, we report that circulating IGFBP-2 concentrations robustly increase after different bariatric procedures in humans, reaching higher levels after biliopancreatic diversion with duodenal switch (BPD-DS) than after Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG). This increase is closely associated with insulin sensitization. In mice and rats, BPD-DS and RYGB operations also increase circulating IGFBP-2 levels, which are not affected by SG or caloric restriction. In mice, Igfbp2 deficiency significantly impairs surgery-induced loss in adiposity and early improvement in insulin sensitivity but does not affect long-term enhancement in glucose homeostasis. This study demonstrates that the modulation of circulating IGFBP-2 may play a role in the early improvement of insulin sensitivity and loss of adiposity brought about by bariatric surgery.
Keywords: BPD-DS; RYGB; bariatric surgery; binding protein; humans; insulin-like growth factor; metabolism; mice; patients; sleeve gastrectomy; type 2 diabetes.
© 2021 The Author(s).
Conflict of interest statement
A.C.C. has received consultant fees from Janssen (2017 and 2018), Novartis (2018), Novo Nordisk (2018), HLS Therapeutics (2019), and Eli Lilly (2020). A.-M.C. has received consultant fees from Pfizer (2017). A.T. received consulting fees from Novo Nordisk and Bausch Health. A.T. and L.B. are the recipients of research grant support from Johnson & Johnson Medical Companies and Medtronic for studies on bariatric surgery and the Research Chair in Bariatric and Metabolic Surgery, respectively, at l’Institut Universitaire de Cardiologie et de Pneumologie de Québec (IUCPQ) and Laval University. All of the other authors reported no competing financial interests in relation to the work described herein.
Figures



References
-
- Poirier P., Cornier M.A., Mazzone T., Stiles S., Cummings S., Klein S., McCullough P.A., Ren Fielding C., Franklin B.A., American Heart Association Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism Bariatric surgery and cardiovascular risk factors: a scientific statement from the American Heart Association. Circulation. 2011;123:1683–1701. - PubMed
-
- Rubino F. Bariatric surgery: effects on glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9:497–507. - PubMed
-
- Abarca-Gómez L., Abdeen Z.A., Hamid Z.A., Abu-Rmeileh N.M., Acosta-Cazares B., Acuin C., Adams R.J., Aekplakorn W., Afsana K., Aguilar-Salinas C.A., NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. - PMC - PubMed
-
- Thomas S., Schauer P. Bariatric surgery and the gut hormone response. Nutr. Clin. Pract. 2010;25:175–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

