This is a preprint.
Previously infected vaccinees broadly neutralize SARS-CoV-2 variants
- PMID: 33948601
- PMCID: PMC8095208
- DOI: 10.1101/2021.04.25.21256049
Previously infected vaccinees broadly neutralize SARS-CoV-2 variants
Abstract
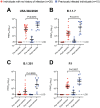
We compared the serum neutralizing antibody titers before and after two doses of the BNT162b2 COVID-19 vaccine in ten individuals who recovered from SARS-CoV-2 infection prior to vaccination to 20 individuals with no history of infection, against clinical isolates of B.1.1.7, B.1.351, P.1, and the original SARS-CoV-2 virus. Vaccination boosted pre-existing levels of anti-SARS-CoV-2 spike antibodies 10-fold in previously infected individuals, but not to levels significantly higher than those of uninfected vaccinees. However, neutralizing antibody titers increased in previously infected vaccinees relative to uninfected vaccinees against every variant tested: 5.2-fold against B.1.1.7, 6.5-fold against B.1.351, 4.3-fold against P.1, and 3.4-fold against original SARS-CoV-2. Our study indicates that a first-generation COVID-19 vaccine provides broad protection from SARS-CoV-2 variants in individuals with previous infection.
Conflict of interest statement
Competing interests Authors declare that they have no competing interests.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
