Soluble P-tau217 reflects amyloid and tau pathology and mediates the association of amyloid with tau
- PMID: 33949133
- PMCID: PMC8185545
- DOI: 10.15252/emmm.202114022
Soluble P-tau217 reflects amyloid and tau pathology and mediates the association of amyloid with tau
Abstract
Alzheimer's disease is characterized by β-amyloid plaques and tau tangles. Plasma levels of phospho-tau217 (P-tau217) accurately differentiate Alzheimer's disease dementia from other dementias, but it is unclear to what degree this reflects β-amyloid plaque accumulation, tau tangle accumulation, or both. In a cohort with post-mortem neuropathological data (N = 88), both plaque and tangle density contributed independently to higher P-tau217, but P-tau217 was not elevated in patients with non-Alzheimer's disease tauopathies (N = 9). Several findings were replicated in a cohort with PET imaging ("BioFINDER-2", N = 426), where β-amyloid and tau PET were independently associated with P-tau217. P-tau217 concentrations correlated with β-amyloid PET (but not tau PET) in early disease stages and with both β-amyloid and (more strongly) tau PET in late disease stages. Finally, P-tau217 mediated the association between β-amyloid and tau in both cohorts, especially for tau outside of the medial temporal lobe. These findings support the hypothesis that plasma P-tau217 concentration is increased by both β-amyloid plaques and tau tangles and is congruent with the hypothesis that P-tau is involved in β-amyloid-dependent formation of neocortical tau tangles.
Keywords: Alzheimer’s disease; amyloid; phosphorylated tau; plasma; tau.
© 2021 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
OH has acquired research support (for the institution) from AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, GE Healthcare, Pfizer, and Roche. In the past 2 years, he has received consultancy/speaker fees from AC Immune, Alzpath, Biogen, Cerveau, and Roche. NMC, ES, SP, SJ, and RS have no disclosures. TGB has had research support from the National Institute on Aging, Michael J Fox Foundation for Parkinson’s Research, and the State of Arizona and, in the past 2 years, has received consultancy and/or speaker fees from Prothena Biosciences and Vivid Genomics. JLD is an employee of Eli Lilly and Company. Remaining co‐authors report no disclosures.
Figures
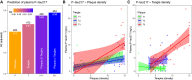
R 2 and AIC for different regression models, using either only amyloid plaque density (“P”), only tangle density (“T”), both plaque and tangle density (“P + T”), or both plaque and tangle density including their interaction term (“PxT”). All models included age and sex as covariates. The panel shows adjusted R 2, together with AIC (above the bars) for each model. We compared R 2 between the models using a bootstrap procedure (N = 1,000 iterations), which showed that the R 2 for the “PxT” model was marginally higher than for the “P + T” model (ΔR 2 = 0.027, 95% CI −0.0034‐0.087) and significantly higher than for the “T” model (ΔR 2 = 0.12, 95% CI 0.04‐0.24) and the “P” model (ΔR 2 = 0.18, 95% CI 0.055‐0.31).
Associations between plasma P‐tau217 and plaque density (stratified by tertiles [T1‐3] of tangle density).
Associations between plasma P‐tau217 and tangle density (stratified by tertiles [T1‐3] of plaque density).
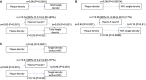
- A–C
Three different mediation models are shown, testing plasma P‐tau217 as a statistical mediator of plaque density on tangle density. Panel (A) shows the mediation of total tangle density (significant mediation, 60% of the direct effect of plaque density on tangle density was explained by plasma P‐tau217). Panel (B) shows the mediation of medial temporal lobe (MTL, entorhinal cortex and hippocampus) tangle density (no mediation). Panel (C) shows the mediation of tangle density in all regions except the MTL (strongest mediation, 77%). The P‐values are from linear regression models used to estimate the mediation effects.

- A–C
Individuals in the neuropathology cohort without tangles in the parietal or frontal lobe and no more than minimal tangle load in the temporal lobe were included in this analysis (N = 42). MTL tangles were defined as tangles in entorhinal cortex plus hippocampus. Relationships between variables were tested in linear regression models, adjusted for age and sex. In these models, plasma P‐tau217 was significantly associated with Aβ plaques (β = 1.64, P < 0.0001) (panel A) but not with MTL tangles (panel B). MTL tangles were not associated with Aβ plaques (panel C). The solid line in panel (A) is the mean effect from the regression model, and the shaded area is the 95% confidence interval of the mean.

- A–C
Individuals in the neuropathology cohort were divided into groups of no significant primary pathology (N = 31), AD (N = 36), PD (N = 13), and others (N = 8) and analyzed together with N = 9 additional subjects with primary non‐AD tauopathies (PSP/CBD). Data are shown for plasma P‐tau217 (panel A), plaque density (panel B), and tangle density (panel C). Plasma P‐tau217 was not elevated in PSP/CBD compared to subjects without significant primary pathology. AD, Alzheimer’s disease; CBD, corticobasal degeneration; PSP, progressive supranuclear palsy; and PD, Parkinson’s disease. The central band of the boxes is medians, and the boxes show interquartile ranges. The whiskers are defined as the smallest (largest) observation greater (less) than or equal to the first (third) quartile minus (plus) 1.5 times the interquartile range.
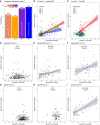
- A
R 2 and AIC for different regression models, using either only Aβ PET (“A”), only tau PET (“T(I‐VI)”), both Aβ and tau PET (“A + T”), or both Aβ and tau PET including their interaction term (“AxT”). All models included age and sex as covariates. The panel shows adjusted R 2, together with AIC (above the bars) for each model. We compared R 2 between the models using a bootstrap procedure (N = 1,000 iterations), verifying that the R 2 for the “AxT” model was higher than for the “A + T” model (ΔR 2 = 0.026, 95% CI 0.010‐0.058), the “T(I‐VI)” model (ΔR 2 = 0.17, 95% CI 0.11‐0.26), and the “A” model (ΔR 2 = 0.14, 95% CI 0.060–0.22).
- B
Associations between plasma P‐tau217 and Aβ PET stratified by tertiles (T) of tau PET (T1: SUVR ≤ 1.05, T2: 1.05 < SUVR ≤ 1.11, T3: 1.11 < SUVR ≤ 3.03).
- C
Associations between plasma P‐tau217 and tau PET stratified by tertiles (T) of Aβ PET (T1: SUVR ≤ 0.468, T2: 0.468 < SUVR ≤ 0.579, T3: 0.579 < SUVR ≤ 1.08).
- D–I
Associations with P‐tau217 across groups of Aβ and tau PET positivity. Cut‐points for Aβ PET (> 0.533 SUVR) and tau PET (> 1.36 SUVR in a ROI corresponding to Braak stages I‐IV) were used to define the groups. Associations are shown for global cortical Aβ PET (panels D‐F) and global cortical tau PET (corresponding to Braak stages I‐VI) (panels G‐I) in individuals classified as negative for both Aβ and tau (N = 264, panels D and G), positive for Aβ only (N = 104, panels E and H), or positive for both Aβ and tau (N = 58, panels F and I). The plots show R 2 and P‐values from linear regression models. No covariates were included in these models, in order to generate R 2‐values for the PET measures alone.

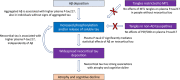
- A, B
Plasma P‐tau217 partly mediated the effect of Aβ PET on tau PET, when using tau PET quantified in Braak stages I‐VI (A, “Tau PET global”, 66% of the direct effect of Aβ PET on tau PET was explained by plasma P‐tau217) or in the medial temporal lobe (B, MTL, entorhinal cortex and hippocampus, 29%). The P‐values are from linear regression models used to estimate the mediation effects.
References
-
- Barthélemy NR, Li Y, Joseph‐Mathurin N, Gordon BA, Hassenstab J, Benzinger TLS, Buckles V, Fagan AM, Perrin RJ, Goate AM et al (2020b) A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer’s disease. Nat Med 26: 398–407 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

