Ir56d-dependent fatty acid responses in Drosophila uncover taste discrimination between different classes of fatty acids
- PMID: 33949306
- PMCID: PMC8169106
- DOI: 10.7554/eLife.67878
Ir56d-dependent fatty acid responses in Drosophila uncover taste discrimination between different classes of fatty acids
Abstract
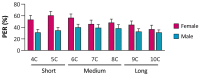
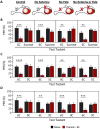
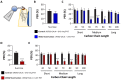
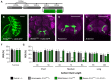
Chemosensory systems are critical for evaluating the caloric value and potential toxicity of food. While animals can discriminate between thousands of odors, much less is known about the discriminative capabilities of taste systems. Fats and sugars represent calorically potent and attractive food sources that contribute to hedonic feeding. Despite the differences in nutritional value between fats and sugars, the ability of the taste system to discriminate between different rewarding tastants is thought to be limited. In Drosophila, taste neurons expressing the ionotropic receptor 56d (IR56d) are required for reflexive behavioral responses to the medium-chain fatty acid, hexanoic acid. Here, we tested whether flies can discriminate between different classes of fatty acids using an aversive memory assay. Our results indicate that flies are able to discriminate medium-chain fatty acids from both short- and long-chain fatty acids, but not from other medium-chain fatty acids. While IR56d neurons are broadly responsive to short-, medium-, and long-chain fatty acids, genetic deletion of IR56d selectively disrupts response to medium-chain fatty acids. Further, IR56d+ GR64f+ neurons are necessary for proboscis extension response (PER) to medium-chain fatty acids, but both IR56d and GR64f neurons are dispensable for PER to short- and long-chain fatty acids, indicating the involvement of one or more other classes of neurons. Together, these findings reveal that IR56d is selectively required for medium-chain fatty acid taste, and discrimination of fatty acids occurs through differential receptor activation in shared populations of neurons. Our study uncovers a capacity for the taste system to encode tastant identity within a taste category.
Keywords: D. melanogaster; fatty acids; neuroscience; sensory plasticity; taste.
© 2021, Brown et al.
Conflict of interest statement
EB, KS, JP, MD, AD, AK No competing interests declared
Figures








Similar articles
-
A subset of sweet-sensing neurons identified by IR56d are necessary and sufficient for fatty acid taste.PLoS Genet. 2017 Nov 9;13(11):e1007059. doi: 10.1371/journal.pgen.1007059. eCollection 2017 Nov. PLoS Genet. 2017. PMID: 29121639 Free PMC article.
-
Molecular basis of fatty acid taste in Drosophila.Elife. 2017 Dec 12;6:e30115. doi: 10.7554/eLife.30115. Elife. 2017. PMID: 29231818 Free PMC article.
-
An expression atlas of variant ionotropic glutamate receptors identifies a molecular basis of carbonation sensing.Nat Commun. 2018 Oct 12;9(1):4252. doi: 10.1038/s41467-018-06453-1. Nat Commun. 2018. PMID: 30315166 Free PMC article.
-
Recent advances in the genetic basis of taste detection in Drosophila.Cell Mol Life Sci. 2020 Mar;77(6):1087-1101. doi: 10.1007/s00018-019-03320-0. Epub 2019 Oct 9. Cell Mol Life Sci. 2020. PMID: 31598735 Free PMC article. Review.
-
Gustatory processing and taste memory in Drosophila.J Neurogenet. 2016 Jun;30(2):112-21. doi: 10.1080/01677063.2016.1185104. J Neurogenet. 2016. PMID: 27328844 Free PMC article. Review.
Cited by
-
Taste triggers a homeostatic temperature control in hungry flies.Elife. 2024 Dec 2;13:RP94703. doi: 10.7554/eLife.94703. Elife. 2024. PMID: 39621014 Free PMC article.
-
Harnessing Insect Chemosensory and Mechanosensory Receptors Involved in Feeding for Precision Pest Management.Life (Basel). 2025 Jan 16;15(1):110. doi: 10.3390/life15010110. Life (Basel). 2025. PMID: 39860050 Free PMC article. Review.
-
Alkaline taste sensation through the alkaliphile chloride channel in Drosophila.Nat Metab. 2023 Mar;5(3):466-480. doi: 10.1038/s42255-023-00765-3. Epub 2023 Mar 20. Nat Metab. 2023. PMID: 36941450 Free PMC article.
-
Taste coding of heavy metal ion-induced avoidance in Drosophila.iScience. 2023 Apr 7;26(5):106607. doi: 10.1016/j.isci.2023.106607. eCollection 2023 May 19. iScience. 2023. PMID: 37128604 Free PMC article.
-
Genomic identification and evolutionary analysis of chemosensory receptor gene families in two Phthorimaea pest species: insights into chemical ecology and host adaptation.BMC Genomics. 2024 May 18;25(1):493. doi: 10.1186/s12864-024-10428-6. BMC Genomics. 2024. PMID: 38762533 Free PMC article.
References
-
- Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderón NC, Esposti F, Borghuis BG, Sun XR, Gordus A, Orger MB, Portugues R, Engert F, Macklin JJ, Filosa A, Aggarwal A, Kerr RA, Takagi R, Kracun S, Shigetomi E, Khakh BS, Baier H, Lagnado L, Wang SS, Bargmann CI, Kimmel BE, Jayaraman V, Svoboda K, Kim DS, Schreiter ER, Looger LL. Optimization of a GCaMP calcium Indicator for neural activity imaging. Journal of Neuroscience. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

