Clinical use of Jak 1 inhibitors for rheumatoid arthritis
- PMID: 33950231
- PMCID: PMC8098107
- DOI: 10.1093/rheumatology/keab265
Clinical use of Jak 1 inhibitors for rheumatoid arthritis
Abstract
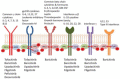
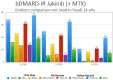
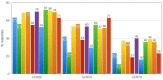
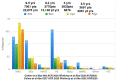
The uptake of Jak inhibitors in the RA space has been among the most rapid in rheumatology, based on the results of comprehensive clinical trial programmes of five agents. Newer generations of Jak inhibitors, like upadacitinib and filgotinib, target Jak 1 selectively with the aim of maximizing efficacy and to improve safety. This article will review the clinical significance of evidence on: (i) Jak 1 selectivity; (ii) efficacy from the SELECT and FINCH clinical trial programmes including patient intolerant or inadequately responding to MTX (MTX-IR) and other csDMARDs patients who are bDMARD-IR) and those using monotherapy when MTX is not tolerated or contraindicated and those treated when methotrexate naive; and (iii) safety from the clinical trial programmes of these two agents will be discussed.
Keywords: Jak 1 selectivity; efficacy; filgotinib; safety; upadacitinib.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures


References
-
- Yu C, Jin S, Wang Y. et al. Remission rate and predictors of remission in patients with rheumatoid arthritis under treat-totarget strategy in real-world studies: a systematic review and meta-analysis. Clin Rheumatol 2019;38:727–38. - PubMed
-
- Taylor PC, Azeez MA, Kiriakidis S.. Filgotinib for the treatment of rheumatoid arthritis. Exp Opin Invest Drugs 2017;26:1181–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

