Engineered microRNA-based regulatory element permits safe high-dose miniMECP2 gene therapy in Rett mice
- PMID: 33950254
- PMCID: PMC8783608
- DOI: 10.1093/brain/awab182
Engineered microRNA-based regulatory element permits safe high-dose miniMECP2 gene therapy in Rett mice
Abstract
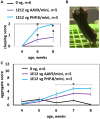
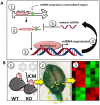
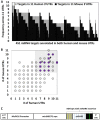
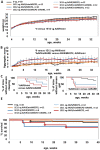
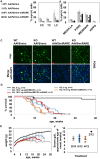
MECP2 gene transfer has been shown to extend the survival of Mecp2-/y knockout mice modelling Rett syndrome, an X-linked neurodevelopmental disorder. However, controlling deleterious overexpression of MECP2 remains the critical unmet obstacle towards a safe and effective gene therapy approach for Rett syndrome. A recently developed truncated miniMECP2 gene has also been shown to be therapeutic after AAV9-mediated gene transfer in knockout neonates. We show that AAV9/miniMECP2 has a similar dose-dependent toxicity profile to that of a published second-generation AAV9/MECP2 vector after treatment in adolescent mice. To overcome that toxicity, we developed a risk-driven viral genome design strategy rooted in high-throughput profiling and genome mining to rationally develop a compact, synthetic microRNA target panel (miR-responsive auto-regulatory element, 'miRARE') to minimize the possibility of miniMECP2 transgene overexpression in the context of Rett syndrome gene therapy. The goal of miRARE is to have a built-in inhibitory element responsive to MECP2 overexpression. The data provided herein show that insertion of miRARE into the miniMECP2 gene expression cassette greatly improved the safety of miniMECP2 gene transfer without compromising efficacy. Importantly, this built-in regulation system does not require any additional exogenous drug application, and no miRNAs are expressed from the transgene cassette. Although broad applications of miRARE have yet to be determined, the design of miRARE suggests a potential use in gene therapy approaches for other dose-sensitive genes.
Keywords: AAV; MECP2; Rett; intrathecal; microRNA.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Chahil G, Bollu PC. Rett syndrome. In: StatPearls [Internet]. StatPearls Publishing; 2019. - PubMed
-
- Van den Veyver IB, Zoghbi HY. Genetic basis of Rett syndrome. Ment Retard Dev Disabil Res Rev . 2002;8(2):82–86. - PubMed
-
- Mnatzakanian GN, Lohi H, Munteanu I, et al. A previously unidentified MECP2 open reading frame defines a new protein isoform relevant to Rett syndrome. Nat Genet . 2004;36(4):339–341. - PubMed
-
- Sinnett SE, Gray SJ. Recent endeavors in MECP2 gene transfer for gene therapy of Rett syndrome. Discov Med . 2017;24(132):153–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

