Galcanezumab Provides Consistent Efficacy Throughout the Dosing Interval Among Patients with Episodic and Chronic Migraine: A Post Hoc Analysis
- PMID: 33950375
- PMCID: PMC8189981
- DOI: 10.1007/s12325-021-01708-8
Galcanezumab Provides Consistent Efficacy Throughout the Dosing Interval Among Patients with Episodic and Chronic Migraine: A Post Hoc Analysis
Abstract
Introduction: The consistency of the treatment effect of galcanezumab throughout the dosing interval is examined in patients with episodic and chronic migraine.
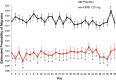
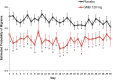
Methods: This study was a post hoc analysis of clinical trial data from episodic (EVOLVE-1; EVOLVE-2; both 6-month duration) and chronic (REGAIN; 3-month duration) migraine double-blind trials evaluating the efficacy of a once-monthly injection of galcanezumab 120 mg relative to placebo. Adults with episodic (placebo, n = 894; galcanezumab, n = 444) or chronic migraine (placebo, n = 558; galcanezumab, n = 278) were included. Mean change from baseline in weekly migraine headache days, averaged across all months for each week of the dosing interval, was compared between groups and within the galcanezumab group during weeks 1 and 4. Additional analyses examined the mean difference from placebo in weekly migraine headache days and a day-by-day analysis.
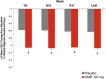
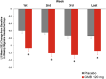
Results: Weekly migraine headache day reduction was significantly greater with galcanezumab relative to placebo every week (P < 0.001) and did not differ during weeks 1 and 4 for those with episodic (P = 0.740) or chronic migraine (P = 0.231) taking galcanezumab. Estimated probabilities of migraine on day 2 and day 30 did not differ for those with episodic (P = 0.61) or chronic migraine (P = 0.616) taking galcanezumab.
Conclusion: This analysis demonstrates once monthly galcanezumab exhibits consistent efficacy throughout the dosing interval among the population of patients with migraine in three clinical trials evaluating the efficacy of galcanezumab. There is no evidence from these trials that the effect of galcanezumab "wears off" at the end of the dosing interval.
Trial registration: ClinicalTrials.gov identifier: EVOLVE-1 (NCT02614183); EVOLVE-2 (NCT02614196); REGAIN (NCT02614261).
Keywords: Chronic; Efficacy; Episodic; Galcanezumab; Migraine; Wear off.
Figures
References
-
- Lipton RB, Araujo AB, Nicholson RA, et al. Patterns of diagnosis, consultation, and treatment of migraine in the US: results of the OVERCOME study. In: 61st Annual Scientific Meeting American Headache Society® 2019 Headache. 2019;59:2–3. 10.1111/head.13549.
-
- Ashina S, Nicholson RA, Buse DC, et al. Identifying barriers to care-seeking, diagnosis, and preventive medication among those with migraine: results of the OVERCOME study [abstract] Headache. 2020;60(S1):127–128.
-
- Nicholson RA, Lipton RB, Reed ML, et al. Characteristics of individuals with migraine who are eligible for novel CGRP monoclonal antibodies: results of the OVERCOME study [abstract] Headache. 2020;60(S1):126–127.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

