Carbazole- and Fluorene-Fused Aza-BODIPYs: NIR Fluorophores with High Brightness and Photostability
- PMID: 33950529
- PMCID: PMC8362076
- DOI: 10.1002/chem.202100965
Carbazole- and Fluorene-Fused Aza-BODIPYs: NIR Fluorophores with High Brightness and Photostability
Abstract
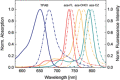
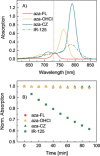
Three new aza-BODIPY dyes incorporating fused fluorene or carbazole moieties have been prepared. The dyes show significant enhancement of photophysical properties compared to the parent 1,3,5,7-tetraphenyl aza-BODIPY (TPAB): a bathochromic shift of the absorption maximum (up to 2700 cm-1 ) and emission maximum (up to 2270 cm-1 ); an almost threefold increase in molar absorption coefficients (to ca. 230 000 M-1 cm-1 ) and a significant increase in the fluorescence quantum yield to 49-66 %. Owing to the combination of these properties, the new aza-BODIPY dyes belong to the brightest NIR dyes reported. The dyes also show excellent photostability. Due to their outstanding properties, the new dyes represent a promising platform for further exploration in biomedical research. A pH indicator containing only one fused carbazole unit was also prepared and shows absorption and emission spectra that are bathochromically shifted by about 110 and 100 nm, respectively, compared to the indicator dye based on the TPAB chromophore.
Keywords: dyes; fluorescence; near-infrared; pH; sensing.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kiyose K., Kojima H., Nagano T., Chem. Asian J. 2008, 3, 506–515.
-
- Qian G., Wang Z. Y., Chem. Asian J. 2010, 5, 1006–1029. - PubMed
-
- Frangioni J., Curr. Opin. Chem. Biol. 2003, 7, 626–634. - PubMed
-
- Shi Z., Han X., Hu W., Bai H., Peng B., Ji L., Fan Q., Li L., Huang W., Chem. Soc. Rev. 2020, 49, 7533–7567. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

