A prospective trial of abiraterone acetate plus prednisone in Black and White men with metastatic castrate-resistant prostate cancer
- PMID: 33951180
- PMCID: PMC9527760
- DOI: 10.1002/cncr.33589
A prospective trial of abiraterone acetate plus prednisone in Black and White men with metastatic castrate-resistant prostate cancer
Abstract
Background: Retrospective analyses of randomized trials suggest that Black men with metastatic castration-resistant prostate cancer (mCRPC) have longer survival than White men. The authors conducted a prospective study of abiraterone acetate plus prednisone to explore outcomes by race.
Methods: This race-stratified, multicenter study estimated radiographic progression-free survival (rPFS) in Black and White men with mCRPC. Secondary end points included prostate-specific antigen (PSA) kinetics, overall survival (OS), and safety. Exploratory analysis included genome-wide genotyping to identify single nucleotide polymorphisms associated with progression in a model incorporating genetic ancestry. One hundred patients self-identified as White (n = 50) or Black (n = 50) were enrolled. Eligibility criteria were modified to facilitate the enrollment of individual Black patients.
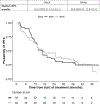
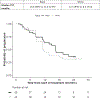
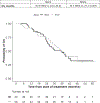
Results: The median rPFS for Black and White patients was 16.6 and 16.8 months, respectively; their times to PSA progression (TTP) were 16.6 and 11.5 months, respectively; and their OS was 35.9 and 35.7 months, respectively. Estimated rates of PSA decline by ≥50% in Black and White patients were 74% and 66%, respectively; and PSA declines to <0.2 ng/mL were 26% and 10%, respectively. Rates of grade 3 and 4 hypertension, hypokalemia, and hyperglycemia were higher in Black men.
Conclusions: Multicenter prospective studies by race are feasible in men with mCRPC but require less restrictive eligibility. Despite higher comorbidity rates, Black patients demonstrated rPFS and OS similar to those of White patients and trended toward greater TTP and PSA declines, consistent with retrospective reports. Importantly, Black men may have higher side-effect rates than White men. This exploratory genome-wide analysis of TTP identified a possible candidate marker of ancestry-dependent treatment outcomes.
Keywords: African American; abiraterone acetate; castration resistant; hormone therapy; metastatic prostate cancer; prednisone; prostate-specific antigen (PSA); race.
© 2021 American Cancer Society.
Conflict of interest statement
CONFLICT OF INTEREST DISCLOSURES
Daniel J. George reports institutional research support from Acerta, Astellas, Bristol-Myers Squibb (BMS), Bayer, Calithera, Exelixis, Janssen Pharma, Myovant, Pfizer, Novartis, and Sanofi-Aventis; and personal fees from the AACR, Astellas, AstraZeneca, Bayer H/C, BMS, Capio Biosciences, Constellation Pharma, EMD Serono, Exelixis, Flatiron, Ipsen, Janssen Pharma, Merck, Michael J Hennessey Associates, Millennium Medical Publishing, Modra Pharma, Myovant Sciences, NCI Genitourinary, Nektar Therapeutics, Pfizer, Physician Education Resource, RevHealth LLC, Propella Therapeutics, Sanofi, UroGPO, UroToday, and Vizuri Health Sciences, outside the submitted work. Susan Halabi serves on the on the Data and Safety Monitoring Boards at Bayer, Eisai, and Ferring and receives funding through the American Society for Clinical Oncology as a statistician on the TAPUR trial. A. Oliver Sartor reports grants from Innocrin, Invitae, Merck, and Sotio; grants and personal fees from Advanced Accelerator Applications, AstraZeneca, Bayer, BMS, Clarity Pharmaceuticals, Clovis Oncology, Constellation, Dendreon, EMD Serono, Endocyte, Fusion, Janssen, Myovant, Myriad, Noria Therapeutics Inc, Novartis, Noxopharm, Pfizer, Progenics, POINT Biopharma, Sanofi, Tenebio, Telix, and Theragnostics; personal fees from Astellas, Blue Earth Diagnostics, Bavarian Nordic, Celgene, and Fusion; and other support from the National Cancer Institute and NRG Oncology, outside the submitted work. Guru P. Sonpavde reports institutional research funding from AstraZeneca, Bayer, Boehringer-Ingelheim, Merck, Pfizer, and Sanofi; grants and personal fees from Gilead, Janssen, and Sanofi; personal fees from Amgen, AstraZeneca, Bicycle Therapeutics, BMS, Dava Oncology, Eisai, Elsevier Practice Update, EMD Serono/Pfizer, Exelixis, G1 Therapeutics, Genentech, Janssen, Medscape, Merck, Novartis, Sanofi, Scholar Rock, and Seattle Genetics/Astellas; personal fees from Clinical Care Options, Onclive, Physicians Education Resource, Research to Practice, and UpToDate; service on steering committees of trials for Bavarian Nordic, BMS, QED, and Seattle Genetics (all unpaid) as well as AstraZeneca and Debiopharm; and other support from Bavarian Nordic and Debiopharm, outside the submitted work. Matthew I. Milowsky reports institutional research funding from Acerta, Amgen, Arvinas, Astelllas, BMS, Clovis Oncology, Constellation, Genentech, Incyte, Innocrin, Inovio, Johnson & Johnson, Merck, Mirati, Pfizer, Regeneron, Roche, Seagen, Syndax, and X4Pharmaceuticals, outside the submitted work. Michael Goodman reports institutional research funding from Janssen, outside the submitted work. Michael R. Harrison reports institutional research funding from Argos, Bayer, BMS, Exelixis, Merck, Pfizer, and Seattle Genetics; and personal fees from BMS, Exelixis, Genentech/Roche, and Janssen, outside the submitted work. Megan McNamara reports institutional research funding from Acerta Pharma, Astellas Pharma, AstraZeneca, Boehringer-Ingelheim, BMS, Cerulean Pharma, Clovis Oncology, Constellation Pharmaceuticals, Incyte, Innocrin Pharma, Inovio Pharmaceuticals, Jounce Therapeutics, MedImmune, Merck, Mirati Therapeutics, Roche/Genentech, Seattle Genetics, Syndax, V Foundation, and X4 Pharmaceuticals; personal fees from Bayer and BioClin Therapeutics; and other institutional support from Asieris. Dadong Zhang reports institutional research funding from AbbVie, Acerta, Astellas, Janssen, Merck, Merrimack, Mirati, Novartis, OmniSeq, Pfizer, PGDx, and Regeneron; personal fees from Amgen, AstraZeneca, Bayer, BMS, Calithera, Dendreon, Exelixis, Foundation Medicine, Genentech/Roche, Genomic Health, IQVIA, Janssen, Larvol, Merck, MUH Associates, Nanorobotics, Pacific Genuity, Pfizer, Pharmacyclics, QED Therapeutics, Sanofi-Aventis, and Seattle Genetics; service on the American Society for Clinical Oncology Clinical Guidelines Committee for Treatment of Metastatic Renal Cell Carcinoma; service on the Aravive Data Safety Monitoring Board; and stock ownership/employment (spouse) from Archimmune Therapeutics and Capio Biosciences, outside the submitted work. Andrew J. Armstrong reports grants from Janssen during the course of the study; institutional research funding from Astellas, AstraZeneca, Bayer, Beigene; BMS, Constellation, Dendreon, Genentech/Roche, Janssen, Merck, Novartis, and Pfizer; personal fees from Astellas/Pfizer, AstraZeneca, Bayer, BMS, Clovis Oncology, Dendreon, Genentech/Roche, and Merck; and personal fees from Clovis Oncology and Janssen, outside the submitted work. The remaining authors made no disclosures.
Figures
References
-
- Surveillance, Epidemiology, and End Results (SEER) Program; National Cancer Institute. Overview of the SEER Program. Accessed January 4, 2021. https://seer.cancer.gov/index.html
-
- Halabi S, Vogelzang NJ, Kornblith AB, et al. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol. 2008;26:2544–2549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

