A high-resolution interactive atlas of the human brainstem using magnetic resonance imaging
- PMID: 33951517
- PMCID: PMC8480283
- DOI: 10.1016/j.neuroimage.2021.118135
A high-resolution interactive atlas of the human brainstem using magnetic resonance imaging
Abstract
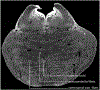
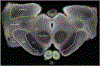
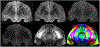
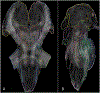
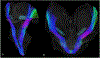
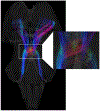
Conventional atlases of the human brainstem are limited by the inflexible, sparsely-sampled, two-dimensional nature of histology, or the low spatial resolution of conventional magnetic resonance imaging (MRI). Postmortem high-resolution MRI circumvents the challenges associated with both modalities. A single human brainstem specimen extending from the rostral diencephalon through the caudal medulla was prepared for imaging after the brain was removed from a 65-year-old male within 24 h of death. The specimen was formalin-fixed for two weeks, then rehydrated and placed in a custom-made MRI compatible tube and immersed in liquid fluorocarbon. MRI was performed in a 7-Tesla scanner with 120 unique diffusion directions. Acquisition time for anatomic and diffusion images were 14 h and 208 h, respectively. Segmentation was performed manually. Deterministic fiber tractography was done using strategically chosen regions of interest and avoidance, with manual editing using expert knowledge of human neuroanatomy. Anatomic and diffusion images were rendered with isotropic resolutions of 50 μm and 200 μm, respectively. Ninety different structures were segmented and labeled, and 11 different fiber bundles were rendered with tractography. The complete atlas is available online for interactive use at https://www.civmvoxport.vm.duke.edu/voxbase/login.php?return_url=%2Fvoxbase%2F. This atlas presents multiple contrasting datasets and selected tract reconstruction with unprecedented resolution for MR imaging of the human brainstem. There are immediate applications in neuroanatomical education, with the potential to serve future applications for neuroanatomical research and enhanced neurosurgical planning through "safe" zones of entry into the human brainstem.
Keywords: Atlas; Brainstem; Diffusion tractography; Human brain; Magnetic resonance imaging.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest None
Figures





References
-
- Afshar F, Watkins ES, Yap JC, 1978. Stereotaxic Atlas of the Human Brainstem and Cerebellar Nuclei. A Variability Study. Raven Press, New York.
-
- Alexander DC, Hubbard PL, Hall MG, Moore EA, Ptito M, Parker GJ, Dyrby TB, 2010. Orientationally invariant indices of axon diameter and density from diffusion MRI. Neuroimage 52, 1374–1389. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

