Phenolic benzotriazoles: a class comparison of toxicokinetics of ultraviolet-light absorbers in male rats
- PMID: 33952035
- PMCID: PMC8883597
- DOI: 10.1080/00498254.2021.1927239
Phenolic benzotriazoles: a class comparison of toxicokinetics of ultraviolet-light absorbers in male rats
Abstract
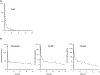
Phenolic benzotriazoles are ultraviolet-light absorbers used in a variety of industrial and consumer applications. We investigated the toxicokinetic behaviour of 9 compounds, covering unsubstituted, monosubstituted, disubstituted, and trisubstituted compounds, following a single gavage (30 and 300 mg/kg) and intravenous (IV) (2.25 mg/kg) administration in male rats.Following IV administration, no distinct pattern in plasma elimination was observed for the compounds with half-lives ranging from 15.4-84.8 h. Systemic exposure parameters, maximum concentration (Cmax) and area under the concentration time curve (AUC), generally increased with the degree of substitution.Following gavage administration, Cmax and AUC of unsubstituted compound were lower compared to the substituted compounds. Cmax and AUC increased ≤7-fold with a 10-fold increase in the dose except for the AUC of the unsubstituted compound where the increase was 30-fold. Plasma elimination half-lives for the class ranged from 1.57 to 192 h with the exception of 30 mg/kg drometrizole.Oral bioavailability was low with ∼ 6% estimated for unsubstituted compound and 12.8-23% for others at 30 mg/kg dose. Bioavailability was lower following administration of the higher dose.Taken collectively, these data point to low oral absorption of phenolic benzotriazoles. The absorption decreased with increasing dose. Substituted compounds may be less metabolized compared to the unsubstituted.
Keywords: Phenolic benzotriazoles; elimination half-life; rats; systemic exposure.
Figures




References
-
- Cantwell MG, Sullivan JC, Burgess RM, 2015. Benzotriazoles: History, Environmental Distribution, and Potential Ecological Effects. ompr. Anal. Chem,.
-
- CIR, 2008. (Cosmetic Ingredient Review) Expert Panel. Amended final report of the safety assessment of drometrizole as used in cosmetics. Int J Toxicol, 27(Suppl 1):63–75. PubMed abstract Internet address: http://www.ncbi.nlm.nih.gov/pubmed/18569163. Last accessed on December, 2020. - PubMed
-
- Council, N.R., 2011. Guide for the Care and Use of Laboratory Animals: Eighth Edition. The National Academies Press, Washington, DC.
-
- Ema M, Fukunishi K, Hirose A, Hirata-Koizumi M, Matsumoto M, Kamata E, 2008. Repeated-dose and reproductive toxicity of the ultraviolet absorber 2-(3’,5’-di- tert-butyl-2’-hydroxyphenyl)-5-chlorobenzotriazole in rats. Drug Chem Toxicol 31, 399–412. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
