Respiratory capacity is twice as important as temperature in explaining patterns of metabolic rate across the vertebrate tree of life
- PMID: 33952516
- PMCID: PMC8099188
- DOI: 10.1126/sciadv.abe5163
Respiratory capacity is twice as important as temperature in explaining patterns of metabolic rate across the vertebrate tree of life
Abstract
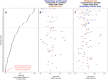
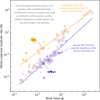
Metabolic rate underlies a wide range of phenomena from cellular dynamics to ecosystem structure and function. Models seeking to statistically explain variation in metabolic rate across vertebrates are largely based on body size and temperature. Unexpectedly, these models overlook variation in the size of gills and lungs that acquire the oxygen needed to fuel aerobic processes. Here, we assess the importance of respiratory surface area in explaining patterns of metabolic rate across the vertebrate tree of life using a novel phylogenetic Bayesian multilevel modeling framework coupled with a species-paired dataset of metabolic rate and respiratory surface area. We reveal that respiratory surface area explains twice as much variation in metabolic rate, compared to temperature, across the vertebrate tree of life. Understanding the combination of oxygen acquisition and transport provides opportunity to understand the evolutionary history of metabolic rate and improve models that quantify the impacts of climate change.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B., Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004).
-
- Gillooly J. F., Brown J. H., West G. B., Savage V. M., Charnov E. L., Effects of size and temperature on metabolic rate. Science 293, 2248–2251 (2001). - PubMed
-
- O’Connor M. P., Kemp S. J., Agosta S. J., Hansen F., Sieg A. E., Wallace B. P., McNair J. N., Dunham A. E., Reconsidering the mechanistic basis of the metabolic theory of ecology. Oikos 116, 1058–1072 (2007).
-
- Barneche D. R., Kulbicki M., Floeter S. R., Friedlander A. M., Maina J., Allen A. P., Scaling metabolism from individuals to reef-fish communities at broad spatial scales. Ecol. Lett. 17, 1067–1076 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

