Development and Verification of a Linked Δ 9-THC/11-OH-THC Physiologically Based Pharmacokinetic Model in Healthy, Nonpregnant Population and Extrapolation to Pregnant Women
- PMID: 33952608
- PMCID: PMC8313051
- DOI: 10.1124/dmd.120.000322
Development and Verification of a Linked Δ 9-THC/11-OH-THC Physiologically Based Pharmacokinetic Model in Healthy, Nonpregnant Population and Extrapolation to Pregnant Women
Abstract
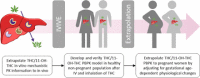
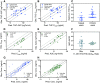
Conducting clinical trials to understand the exposure risk/benefit relationship of cannabis use is not always feasible. Alternatively, physiologically based pharmacokinetic (PBPK) models can be used to predict exposure of the psychoactive cannabinoid (-)-Δ9-tetrahydrocannabinol (THC) and its active metabolite 11-hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC). Here, we first extrapolated in vitro mechanistic pharmacokinetic information previously quantified to build a linked THC/11-OH-THC PBPK model and verified the model with observed data after intravenous and inhalation administration of THC in a healthy, nonpregnant population. The in vitro to in vivo extrapolation of both THC and 11-OH-THC disposition was successful. The inhalation bioavailability (Finh) of THC after inhalation was higher in chronic versus casual cannabis users (Finh = 0.35 and 0.19, respectively). Sensitivity analysis demonstrated that 11-OH-THC but not THC exposure was sensitive to alterations in hepatic intrinsic clearance of the respective compound. Next, we extrapolated the linked THC/11-OH-THC PBPK model to pregnant women. Simulations showed that THC plasma area under the curve (AUC) does not change during pregnancy, but 11-OH-THC plasma AUC decreases by up to 41%. Using a maternal-fetal PBPK model, maternal and fetal THC serum concentrations were simulated and compared with the observed THC serum concentrations in pregnant women at term. To recapitulate the observed THC fetal serum concentrations, active placental efflux of THC needed to be invoked. In conclusion, we built and verified a linked THC/11-OH-THC PBPK model in healthy nonpregnant population and demonstrated how this mechanistic physiologic and pharmacokinetic platform can be extrapolated to a special population, such as pregnant women. SIGNIFICANCE STATEMENT: Although the pharmacokinetics of cannabinoids have been extensively studied clinically, limited mechanistic pharmacokinetic models exist. Here, we developed and verified a physiologically based pharmacokinetic (PBPK) model for (-)-Δ9-tetrahydrocannabinol (THC) and its active metabolite, 11-hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC). The PBPK model was verified in healthy, nonpregnant population after intravenous and inhalation administration of THC, and then extrapolated to pregnant women. The THC/11-OH-THC PBPK model can be used to predict exposure in special populations, predict drug-drug interactions, or impact of genetic polymorphism.
Copyright © 2021 by The American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
The authors report no conflicts of interest.
Figures


References
-
- Anderson GD (2005) Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet 44:989–1008. - PubMed
-
- Bailey JR, Cunny HC, Paule MG, Slikker W Jr (1987) Fetal disposition of delta 9-tetrahydrocannabinol (THC) during late pregnancy in the rhesus monkey. Toxicol Appl Pharmacol 90:315–321. - PubMed
-
- Barkus EMorrison PDVuletic DDickson JCEll PJPilowsky LSBrenneisen RHolt DWPowell JKapur S, et al. (2011) Does intravenous Δ9-tetrahydrocannabinol increase dopamine release? A SPET study. J Psychopharmacol 25:1462–1468. - PubMed
-
- Barnett G, Chiang CW, Perez-Reyes M, Owens SM (1982) Kinetic study of smoking marijuana. J Pharmacokinet Biopharm 10:495–506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

