Serine Protease HTRA1 as a Novel Target Antigen in Primary Membranous Nephropathy
- PMID: 33952630
- PMCID: PMC8425645
- DOI: 10.1681/ASN.2020101395
Serine Protease HTRA1 as a Novel Target Antigen in Primary Membranous Nephropathy
Abstract
Background: Identification of target antigens PLA2R, THSD7A, NELL1, or Semaphorin-3B can explain the majority of cases of primary membranous nephropathy (MN). However, target antigens remain unidentified in 15%-20% of patients.
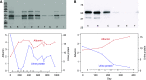
Methods: A multipronged approach, using traditional and modern technologies, converged on a novel target antigen, and capitalized on the temporal variation in autoantibody titer for biomarker discovery. Immunoblotting of human glomerular proteins followed by differential immunoprecipitation and mass spectrometric analysis was complemented by laser-capture microdissection followed by mass spectrometry, elution of immune complexes from renal biopsy specimen tissue, and autoimmune profiling on a protein fragment microarray.
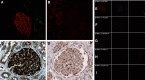
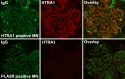
Results: These approaches identified serine protease HTRA1 as a novel podocyte antigen in a subset of patients with primary MN. Sera from two patients reacted by immunoblotting with a 51-kD protein within glomerular extract and with recombinant human HTRA1, under reducing and nonreducing conditions. Longitudinal serum samples from these patients seemed to correlate with clinical disease activity. As in PLA2R- and THSD7A- associated MN, anti-HTRA1 antibodies were predominantly IgG4, suggesting a primary etiology. Analysis of sera collected during active disease versus remission on protein fragment microarrays detected significantly higher titers of anti-HTRA1 antibody in active disease. HTRA1 was specifically detected within immune deposits of HTRA1-associated MN in 14 patients identified among three cohorts. Screening of 118 "quadruple-negative" (PLA2R-, THSD7A-, NELL1-, EXT2-negative) patients in a large repository of MN biopsy specimens revealed a prevalence of 4.2%.
Conclusions: Conventional and more modern techniques converged to identify serine protease HTRA1 as a target antigen in MN.
Keywords: membranous nephropathy; nephrotic syndrome; podocyte.
Copyright © 2021 by the American Society of Nephrology.
Figures






References
-
- McGrogan A, Franssen CF, de Vries CS: The incidence of primary glomerulonephritis worldwide: A systematic review of the literature. Nephrol Dial Transplant 26: 414–430, 2011 - PubMed
-
- Sethi S, Debiec H, Madden B, Charlesworth MC, Morelle J, Gross L, et al.: Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int 97: 163–174, 2020 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

