iPSC-endothelial cell phenotypic drug screening and in silico analyses identify tyrphostin-AG1296 for pulmonary arterial hypertension
- PMID: 33952674
- PMCID: PMC8762958
- DOI: 10.1126/scitranslmed.aba6480
iPSC-endothelial cell phenotypic drug screening and in silico analyses identify tyrphostin-AG1296 for pulmonary arterial hypertension
Abstract
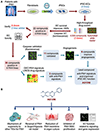
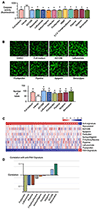
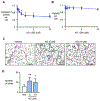
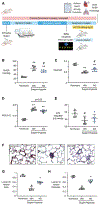
Pulmonary arterial hypertension (PAH) is a progressive disorder leading to occlusive vascular remodeling. Current PAH therapies improve quality of life but do not reverse structural abnormalities in the pulmonary vasculature. Here, we used high-throughput drug screening combined with in silico analyses of existing transcriptomic datasets to identify a promising lead compound to reverse PAH. Induced pluripotent stem cell-derived endothelial cells generated from six patients with PAH were exposed to 4500 compounds and assayed for improved cell survival after serum withdrawal using a chemiluminescent caspase assay. Subsequent validation of caspase activity and improved angiogenesis combined with data analyses using the Gene Expression Omnibus and Library of Integrated Network-Based Cellular Signatures databases revealed that the lead compound AG1296 was positively associated with an anti-PAH gene signature. AG1296 increased abundance of bone morphogenetic protein receptors, downstream signaling, and gene expression and suppressed PAH smooth muscle cell proliferation. AG1296 induced regression of PA neointimal lesions in lung organ culture and PA occlusive changes in the Sugen/hypoxia rat model and reduced right ventricular systolic pressure. Moreover, AG1296 improved vascular function and BMPR2 signaling and showed better correlation with the anti-PAH gene signature than other tyrosine kinase inhibitors. Specifically, AG1296 up-regulated small mothers against decapentaplegic (SMAD) 1/5 coactivators, cAMP response element-binding protein 3 (CREB3), and CREB5: CREB3 induced inhibitor of DNA binding 1 and downstream genes that improved vascular function. Thus, drug discovery for PAH can be accelerated by combining phenotypic screening with in silico analyses of publicly available datasets.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures



References
-
- Diebold I, Hennigs JK, Miyagawa K, Li CG, Nickel NP, Kaschwich M, Cao A, Wang L, Reddy S, Chen PI, Nakahira K, Alcazar MA, Hopper RK, Ji L, Feldman BJ, Rabinovitch M, BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab 21, 596–608 (2015). - PMC - PubMed
-
- Rhodes CJ, Im H, Cao A, Hennigs JK, Wang L, Sa S, Chen PI, Nickel NP, Miyagawa K, Hopper RK, Tojais NF, Li CG, Gu M, Spiekerkoetter E, Xian Z, Chen R, Zhao M, Kaschwich M, Del Rosario PA, Bernstein D, Zamanian RT, Wu JC, Snyder MP, Rabinovitch M, RNA Sequencing Analysis Detection of a Novel Pathway of Endothelial Dysfunction in Pulmonary Arterial Hypertension. American journal of respiratory and critical care medicine 192, 356–366 (2015). - PMC - PubMed
-
- Gu M, Shao NY, Sa S, Li D, Termglinchan V, Ameen M, Karakikes I, Sosa G, Grubert F, Lee J, Cao A, Taylor S, Ma Y, Zhao Z, Chappell J, Hamid R, Austin ED, Gold JD, Wu JC, Snyder MP, Rabinovitch M, Patient-Specific iPSC-Derived Endothelial Cells Uncover Pathways that Protect against Pulmonary Hypertension in BMPR2 Mutation Carriers. Cell Stem Cell 20, 490–504 e495 (2017). - PMC - PubMed
-
- Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, Rabinovitch M, An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest 118, 1846–1857 (2008). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

