Tau forms oligomeric complexes on microtubules that are distinct from tau aggregates
- PMID: 33952699
- PMCID: PMC8126857
- DOI: 10.1073/pnas.2021461118
Tau forms oligomeric complexes on microtubules that are distinct from tau aggregates
Abstract
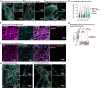
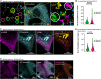
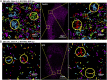
Tau is a microtubule-associated protein, which promotes neuronal microtubule assembly and stability. Accumulation of tau into insoluble aggregates known as neurofibrillary tangles (NFTs) is a pathological hallmark of several neurodegenerative diseases. The current hypothesis is that small, soluble oligomeric tau species preceding NFT formation cause toxicity. However, thus far, visualizing the spatial distribution of tau monomers and oligomers inside cells under physiological or pathological conditions has not been possible. Here, using single-molecule localization microscopy, we show that tau forms small oligomers on microtubules ex vivo. These oligomers are distinct from those found in cells exhibiting tau aggregation and could be precursors of aggregated tau in pathology. Furthermore, using an unsupervised shape classification algorithm that we developed, we show that different tau phosphorylation states are associated with distinct tau aggregate species. Our work elucidates tau's nanoscale composition under nonaggregated and aggregated conditions ex vivo.
Keywords: protein aggregation; super-resolution microscopy; tau.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Weissmann C., et al. ., Microtubule binding and trapping at the tip of neurites regulate tau motion in living neurons. Traffic 10, 1655–1668 (2009). - PubMed
-
- Qiang L., et al. ., Tau does not stabilize axonal microtubules but rather enables them to have long labile domains. Curr. Biol. 28, 2181–2189.e4 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

