Leukaemia: a model metastatic disease
- PMID: 33953370
- PMCID: PMC8722462
- DOI: 10.1038/s41568-021-00355-z
Leukaemia: a model metastatic disease
Abstract
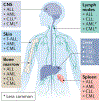
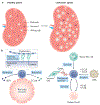
In contrast to solid cancers, which often require genetic modifications and complex cellular reprogramming for effective metastatic dissemination, leukaemic cells uniquely possess the innate ability for migration and invasion. Dedifferentiated, malignant leukocytes retain the benign leukocytes' capacity for cell motility and survival in the circulation, while acquiring the potential for rapid and uncontrolled cell division. For these reasons, leukaemias, although not traditionally considered as metastatic diseases, are in fact models of highly efficient metastatic spread. Accordingly, they are often aggressive and challenging diseases to treat. In this Perspective, we discuss the key molecular processes that facilitate metastasis in a variety of leukaemic subtypes, the clinical significance of leukaemic invasion into specific tissues and the current pipeline of treatments targeting leukaemia metastasis.
Figures

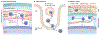
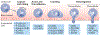
References
-
- Lapidot T et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367, 645–648 (1994). - PubMed
-
- Vetrie D, Helgason GV & Copland M The leukaemia stem cell: similarities, differences and clinical prospects in CML and AML. Nat. Rev. Cancer 20, 158–173 (2020). - PubMed
-
- Jin L, Hope KJ, Zhai Q, Smadja-Joffe F & Dick JE Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat. Med 12, 1167–1174 (2006). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

