Fast odour dynamics are encoded in the olfactory system and guide behaviour
- PMID: 33953395
- PMCID: PMC7611658
- DOI: 10.1038/s41586-021-03514-2
Fast odour dynamics are encoded in the olfactory system and guide behaviour
Abstract
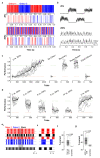
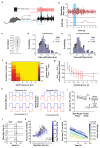
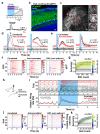
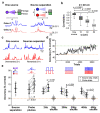
Odours are transported in turbulent plumes, which result in rapid concentration fluctuations1,2 that contain rich information about the olfactory scenery, such as the composition and location of an odour source2-4. However, it is unclear whether the mammalian olfactory system can use the underlying temporal structure to extract information about the environment. Here we show that ten-millisecond odour pulse patterns produce distinct responses in olfactory receptor neurons. In operant conditioning experiments, mice discriminated temporal correlations of rapidly fluctuating odours at frequencies of up to 40 Hz. In imaging and electrophysiological recordings, such correlation information could be readily extracted from the activity of mitral and tufted cells-the output neurons of the olfactory bulb. Furthermore, temporal correlation of odour concentrations5 reliably predicted whether odorants emerged from the same or different sources in naturalistic environments with complex airflow. Experiments in which mice were trained on such tasks and probed using synthetic correlated stimuli at different frequencies suggest that mice can use the temporal structure of odours to extract information about space. Thus, the mammalian olfactory system has access to unexpectedly fast temporal features in odour stimuli. This endows animals with the capacity to overcome key behavioural challenges such as odour source separation5, figure-ground segregation6 and odour localization7 by extracting information about space from temporal odour dynamics.
Conflict of interest statement
The authors declare no competing financial interests.
Figures










References
-
- Fackrell J, Robins A. Concentration fluctuations and fluxes in plumes from point sources in a turbulent boundary layer. J Fluid Mech. 1982;117:1–26.
-
- Mylne KR, Mason PJ. Concentration fluctuation measurements in a dispersing plume at a range of up to 1000 m. Q J R Meteorol Soc. 1991;117:177–206.
-
- Schmuker M, Bahr V, Huerta R. Exploiting plume structure to decode gas source distance using metal-oxide gas sensors. Sensors Actuators B Chem. 2016;235:636–646.
-
- Murlis J, Elkington JS, Cardé RT. Odor Plumes And How Insects Use Them. Annu Rev Entomol. 1992;37:505–532.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

