Differences in activation of intracellular signaling in primary human retinal endothelial cells between isoforms of VEGFA 165
- PMID: 33953532
- PMCID: PMC8092446
Differences in activation of intracellular signaling in primary human retinal endothelial cells between isoforms of VEGFA 165
Abstract
Purpose: There are reports that a b-isoform of vascular endothelial growth factor-A 165 (VEGFA165b) is predominant in normal human vitreous, switching to the a-isoform (VEGFA165a) in the vitreous of some diseased eyes. Although these isoforms appear to have a different ability to activate the VEGF receptor 2 (VEGFR2) in various endothelial cells, the nature of their ability to activate intracellular signaling pathways is not fully characterized, especially in retinal endothelial cells. We determined their activation potential for two key intracellular signaling pathways (MAPK, AKT) over complete dose-response curves and compared potential effects on the expression of several VEGFA165 target genes in primary human retinal microvascular endothelial cells (HRMECs).
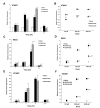
Methods: To determine full dose-response curves for the activation of MAPK (ERK1/2), AKT, and VEGFR2, direct in-cell western assays were developed using primary HRMECs. Potential differences in dose-response effects on gene expression markers related to endothelial cell and leukocyte adhesion (ICAM1, VCAM1, and SELE) and tight junctions (CLDN5 and OCLN) were tested with quantitative PCR.
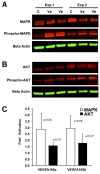
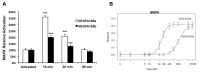
Results: Activation dose-response analysis revealed much stronger activation of MAPK, AKT, and VEGFR2 by the a-isoform at lower doses. MAPK activation in primary HRMECs displayed a sigmoidal dose-response to a range of VEGFA 165 a concentrations spanning 10-250 pM, which shifted higher into the 100-5,000 pM range with VEGFA 165 b. Similar maximum activation of MAPK was achieved by both isoforms at high concentrations. Maximum activation of AKT by VEGFA 165 b was only half of the maximum activation from VEGFA 165 a. At a lower intermediate dose, where VEGFA 165 a activated intracellular signaling stronger than VEGFA 165 b, the changes in VEGFA target gene expression were generally greater with VEGFA 165 a.
Conclusions: In primary HRMECs, VEGFA 165 a could maximally activate MAPK and AKT at lower concentrations where VEGFA 165 b had relatively little effect. The timing for maximum activation of MAPK was similar for the isoforms, which is different from that reported for non-retinal endothelial cells. Although differences in VEGFA 165 a and VEGFA 165 b are limited to the sequence of their six C-terminal six amino acids, this results in a large difference in their ability to activate at least two key intracellular signaling pathways and VEGF-target gene expression in primary human retinal endothelial cells.
Copyright © 2021 Molecular Vision.
Figures



References
-
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–7. - PubMed
-
- Miller JW, Adamis AP, Aiello LP. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab Rev. 1997;13:37–50. - PubMed
-
- Robbins SG, Conaway JR, Ford BL, Roberto KA, Penn JS. Detection of vascular endothelial growth factor (VEGF) protein in vascular and non-vascular cells of the normal and oxygen-injured rat retina. Growth Factors. 1997;14:229–41. - PubMed
-
- Amadio M, Govoni S, Pascale A. Targeting VEGF in eye neovascularization: What’s new?: A comprehensive review on current therapies and oligonucleotide-based interventions under development. Pharmacol Res. 2016;103:253–69. - PubMed
-
- Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R, Couvillion S, Nasir MA, Rabena MD, Le K, Maia M, Visich JE. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular amd. Br J Ophthalmol. 2014;98:1636–41. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

