From the Role of Microbiota in Gut-Lung Axis to SARS-CoV-2 Pathogenesis
- PMID: 33953641
- PMCID: PMC8059477
- DOI: 10.1155/2021/6611222
From the Role of Microbiota in Gut-Lung Axis to SARS-CoV-2 Pathogenesis
Abstract
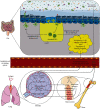
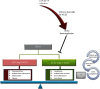
Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) is responsible for the outbreak of a new viral respiratory infection. It has been demonstrated that the microbiota has a crucial role in establishing immune responses against respiratory infections, which are controlled by a bidirectional cross-talk, known as the "gut-lung axis." The effects of microbiota on antiviral immune responses, including dendritic cell (DC) function and lymphocyte homing in the gut-lung axis, have been reported in the recent literature. Additionally, the gut microbiota composition affects (and is affected by) the expression of angiotensin-converting enzyme-2 (ACE2), which is the main receptor for SARS-CoV-2 and contributes to regulate inflammation. Several studies demonstrated an altered microbiota composition in patients infected with SARS-CoV-2, compared to healthy individuals. Furthermore, it has been shown that vaccine efficacy against viral respiratory infection is influenced by probiotics pretreatment. Therefore, the importance of the gut microbiota composition in the lung immune system and ACE2 expression could be valuable to provide optimal therapeutic approaches for SARS-CoV-2 and to preserve the symbiotic relationship of the microbiota with the host.
Copyright © 2021 Sara Ahmadi Badi et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Limborg M. T., Heeb P. Coevolution of Hosts and Their Microbiome. Multidisciplinary Digital Publishing Institute; 2018.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

