Pure Total Flavonoids From Citrus Protect Against Nonsteroidal Anti-inflammatory Drug-Induced Small Intestine Injury by Promoting Autophagy in vivo and in vitro
- PMID: 33953669
- PMCID: PMC8090934
- DOI: 10.3389/fphar.2021.622744
Pure Total Flavonoids From Citrus Protect Against Nonsteroidal Anti-inflammatory Drug-Induced Small Intestine Injury by Promoting Autophagy in vivo and in vitro
Abstract
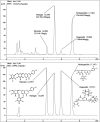
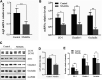
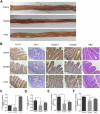
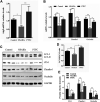
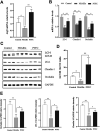
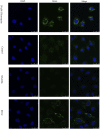
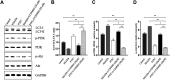
Small intestine injury is an adverse effect of non-steroidal anti-inflammatory drugs (NSAIDs) that urgently needs to be addressed for their safe application. Although pure total flavonoids from citrus (PTFC) have been marketed for the treatment of digestive diseases, their effects on small intestine injury and the underlying mechanism of action remain unknown. This study aimed to investigate the potential role of autophagy in the mechanism of NSAID (diclofenac)-induced intestinal injury in vivo and in vitro and to demonstrate the protective effects of PTFC against NSAID-induced small intestine disease. The results of qRT-PCR, western blotting, and immunohistochemistry showed that the expression levels of autophagy-related 5 (Atg5), light chain 3 (LC3)-II, and tight junction (TJ) proteins ZO-1, claudin-1, and occludin were decreased in rats with NSAID-induced small intestine injury and diclofenac-treated IEC-6 cells compared with the control groups. In the PTFC group, Atg5 and LC3-II expression, TJ protein expression, and the LC3-II/LC3-I ratio increased. Furthermore, the mechanism by which PTFC promotes autophagy in vivo and in vitro was evaluated by western blotting. Expression levels of p-PI3K and p-Akt increased in the intestine disease-induced rat model group compared with the control, but decreased in the PTFC group. Autophagy of IEC-6 cells was upregulated after treatment with a PI3K inhibitor, and the upregulation was significantly more after PTFC treatment, suggesting PTFC promoted autophagy through the PI3K/Akt signaling pathway. In conclusion, PTFC protected intestinal barrier integrity by promoting autophagy, which demonstrates its potential as a therapeutic candidate for NSAID-induced small intestine injury.
Keywords: PI3K-AKT pathway; autophagy; non-steroidal anti-inflammatory drugs; pure total flavonoids from citrus; small intestine injury.
Copyright © 2021 Chen, Jiang, Chao, Hong, Cao and Zhang.
Conflict of interest statement
The author JJ was employed by the Zhejiang You-du Biotech Limited Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Berberine mitigates diclofenac-induced intestinal mucosal mechanical barrier dysfunction through the restoration of autophagy by inhibiting exosome-mediated lncRNA H19.Inflammopharmacology. 2024 Aug;32(4):2525-2540. doi: 10.1007/s10787-024-01487-y. Epub 2024 May 17. Inflammopharmacology. 2024. PMID: 38758516
-
Pure total flavonoids from Citrus ameliorate NSAIDs-induced intestinal mucosal injury via regulation of exosomal LncRNA H19 and protective autophagy.Heliyon. 2024 Apr 26;10(9):e29797. doi: 10.1016/j.heliyon.2024.e29797. eCollection 2024 May 15. Heliyon. 2024. PMID: 38707329 Free PMC article.
-
Pure total flavonoids from citrus improve nonalcoholic steatohepatitis liver inflammatory responses by regulating the CCL2/CCR2-PI3K-Akt signal transduction pathway.Anat Rec (Hoboken). 2023 Dec;306(12):3169-3177. doi: 10.1002/ar.25117. Epub 2022 Dec 9. Anat Rec (Hoboken). 2023. PMID: 36484169
-
Autophagy and tight junction proteins in the intestine and intestinal diseases.Anim Nutr. 2015 Sep;1(3):123-127. doi: 10.1016/j.aninu.2015.08.014. Epub 2015 Sep 1. Anim Nutr. 2015. PMID: 29767173 Free PMC article. Review.
-
The new exploration of pure total flavonoids extracted from Citrus maxima (Burm.) Merr. as a new therapeutic agent to bring health benefits for people.Front Nutr. 2022 Oct 6;9:958329. doi: 10.3389/fnut.2022.958329. eCollection 2022. Front Nutr. 2022. PMID: 36276813 Free PMC article. Review.
Cited by
-
Postbiotic muramyl dipeptide alleviates colitis via activating autophagy in intestinal epithelial cells.Front Pharmacol. 2022 Nov 23;13:1052644. doi: 10.3389/fphar.2022.1052644. eCollection 2022. Front Pharmacol. 2022. PMID: 36506547 Free PMC article.
-
Phytochemical Compounds as Promising Therapeutics for Intestinal Fibrosis in Inflammatory Bowel Disease: A Critical Review.Nutrients. 2024 Oct 25;16(21):3633. doi: 10.3390/nu16213633. Nutrients. 2024. PMID: 39519465 Free PMC article. Review.
-
NSAID-Associated Small Intestinal Injury: An Overview From Animal Model Development to Pathogenesis, Treatment, and Prevention.Front Pharmacol. 2022 Feb 9;13:818877. doi: 10.3389/fphar.2022.818877. eCollection 2022. Front Pharmacol. 2022. PMID: 35222032 Free PMC article. Review.
-
Citrus aurantium 'Changshan-huyou'-An ethnopharmacological and phytochemical review.Front Pharmacol. 2022 Sep 2;13:983470. doi: 10.3389/fphar.2022.983470. eCollection 2022. Front Pharmacol. 2022. PMID: 36133822 Free PMC article. Review.
-
Berberine mitigates diclofenac-induced intestinal mucosal mechanical barrier dysfunction through the restoration of autophagy by inhibiting exosome-mediated lncRNA H19.Inflammopharmacology. 2024 Aug;32(4):2525-2540. doi: 10.1007/s10787-024-01487-y. Epub 2024 May 17. Inflammopharmacology. 2024. PMID: 38758516
References
-
- Bondonno N. P., Bondonno C. P., Blekkenhorst L. C., Considine M. J., Maghzal G., Stocker R., et al. (2018). Flavonoid-rich apple improves endothelial function in individuals at risk for cardiovascular disease: a randomized controlled clinical trial. Mol. Nutr. Food Res. 62, 1700674–1700710. 10.1002/mnfr.201700674 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

