A Comparison Between Recombinant Listeria GAPDH Proteins and GAPDH Encoding mRNA Conjugated to Lipids as Cross-Reactive Vaccines for Listeria, Mycobacterium, and Streptococcus
- PMID: 33953709
- PMCID: PMC8092121
- DOI: 10.3389/fimmu.2021.632304
A Comparison Between Recombinant Listeria GAPDH Proteins and GAPDH Encoding mRNA Conjugated to Lipids as Cross-Reactive Vaccines for Listeria, Mycobacterium, and Streptococcus
Abstract
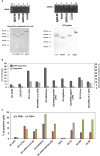
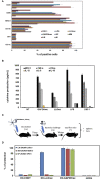
Cross-reactive vaccines recognize common molecular patterns in pathogens and are able to confer broad spectrum protection against different infections. Antigens common to pathogenic bacteria that induce broad immune responses, such as the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) of the genera Listeria, Mycobacterium, or Streptococcus, whose sequences present more than 95% homology at the N-terminal GAPDH1-22 peptide, are putative candidates for universal vaccines. Here, we explore vaccine formulations based on dendritic cells (DC) loaded with two molecular forms of Listeria monocytogenes GAPDH (LM-GAPDH), such as mRNA carriers or recombinant proteins, and compare them with the same molecular forms of three other antigens used in experimental vaccines, listeriolysin O of Listeria monocytogeness, Ag85A of Mycobacterium marinum, and pneumolysin of Streptococcus pneumoniae. DC loaded with LM-GAPDH recombinant proteins proved to be the safest and most immunogenic vaccine vectors, followed by mRNA encoding LM-GAPDH conjugated to lipid carriers. In addition, macrophages lacked sufficient safety as vaccines for all LM-GAPDH molecular forms. The ability of DC loaded with LM-GAPDH recombinant proteins to induce non-specific DC activation explains their adjuvant potency and their capacity to trigger strong CD4+ and CD8+ T cell responses explains their high immunogenicity. Moreover, their capacity to confer protection in vaccinated mice against challenges with L. monocytogenes, M. marinum, or S. pneumoniae validated their efficiency as cross-reactive vaccines. Cross-protection appears to involve the induction of high percentages of GAPDH1-22 specific CD4+ and CD8+ T cells stained for intracellular IFN-γ, and significant levels of peptide-specific antibodies in vaccinated mice. We concluded that DC vaccines loaded with L. monocytogenes GAPDH recombinant proteins are cross-reactive vaccines that seem to be valuable tools in adult vaccination against Listeria, Mycobacterium, and Streptococcus taxonomic groups.
Keywords: cross-reactive vaccines; glyceraldehyde-3-phosphate-dehydrogenase; innate immunity; listeriosis; pneumonia; tuberculosis.
Copyright © 2021 Teran-Navarro, Salcines-Cuevas, Calderon-Gonzalez, Tobes, Calvo-Montes, Pérez-Del Molino Bernal, Yañez-Diaz, Fresno and Alvarez-Dominguez.
Conflict of interest statement
MF was employed by company DIOMUNE S.L. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Calderon-Gonzalez R, Tobes R, Pareja E, Frande-Cabanes E, Alaez-Alvarez L, Petrovsky N, et al. Identification and characterisation of T-cell epitopes for incorporation into dendritic cell-delivered Listeria vaccines. J Immunol Methods. (2015) 424:111–9. 10.1016/j.jim.2015.05.009 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

