DNA-polymer conjugates via the graft-through polymerisation of native DNA in water
- PMID: 33954310
- PMCID: PMC8168459
- DOI: 10.1039/d0cc08008j
DNA-polymer conjugates via the graft-through polymerisation of native DNA in water
Abstract
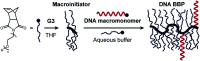
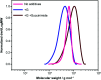
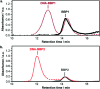
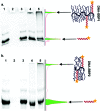
The direct, graft-through, ring-opening metathesis polymerisation (ROMP) of unprotected DNA macromonomers is reported. By tuning the polymerisation conditions, good control is achieved, enabling the rapid and efficient synthesis of DNA-containing bottlebrush copolymers, without the need for protection of the DNA bases.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Water-soluble polyphosphonate-based bottlebrush copolymers via aqueous ring-opening metathesis polymerization.Chem Sci. 2023 Sep 26;14(40):11273-11282. doi: 10.1039/d3sc02649c. eCollection 2023 Oct 18. Chem Sci. 2023. PMID: 37860667 Free PMC article.
-
Preparation of Bottlebrush Polymers via a One-Pot Ring-Opening Polymerization (ROP) and Ring-Opening Metathesis Polymerization (ROMP) Grafting-Through Strategy.Macromol Rapid Commun. 2016 Apr;37(7):616-21. doi: 10.1002/marc.201500672. Epub 2016 Feb 4. Macromol Rapid Commun. 2016. PMID: 26847467
-
ROMP of Macromonomers Prepared by ROMP: Expanding Access to Complex, Functional Bottlebrush Polymers.J Am Chem Soc. 2025 Jan 29;147(4):3855-3865. doi: 10.1021/jacs.4c17151. Epub 2025 Jan 14. J Am Chem Soc. 2025. PMID: 39808775
-
Tandem Ring-Opening Metathesis - Vinyl Insertion Polymerization: Fundamentals and Application to Functional Polyolefins.Macromol Rapid Commun. 2017 Mar;38(6). doi: 10.1002/marc.201600672. Epub 2017 Feb 7. Macromol Rapid Commun. 2017. PMID: 28169470 Review.
-
Developing new methods for the mono-end functionalization of living ring opening metathesis polymers.Chimia (Aarau). 2012;66(3):99-103. doi: 10.2533/chimia.2012.99. Chimia (Aarau). 2012. PMID: 22546252 Review.
Cited by
-
Exploring the Structural Diversity of DNA Bottlebrush Polymers Using an Oligonucleotide Macromonomer Approach.Macromolecules. 2022 Mar 22;55(6):2235-2242. doi: 10.1021/acs.macromol.1c02624. Epub 2022 Mar 1. Macromolecules. 2022. PMID: 36187461 Free PMC article.
-
Water-Accelerated Decomposition of Olefin Metathesis Catalysts.ACS Catal. 2023 Jan 3;13(2):1097-1102. doi: 10.1021/acscatal.2c05573. eCollection 2023 Jan 20. ACS Catal. 2023. PMID: 36714054 Free PMC article.
-
Olefin Metathesis in Water: Speciation of a Leading Water-Soluble Catalyst Pinpoints Challenges and Opportunities for Chemical Biology.J Am Chem Soc. 2025 Mar 19;147(11):9441-9448. doi: 10.1021/jacs.4c16700. Epub 2025 Mar 7. J Am Chem Soc. 2025. PMID: 40053839 Free PMC article.
-
Water-soluble polyphosphonate-based bottlebrush copolymers via aqueous ring-opening metathesis polymerization.Chem Sci. 2023 Sep 26;14(40):11273-11282. doi: 10.1039/d3sc02649c. eCollection 2023 Oct 18. Chem Sci. 2023. PMID: 37860667 Free PMC article.
-
Biomass RNA for the Controlled Synthesis of Degradable Networks by Radical Polymerization.ACS Nano. 2023 Nov 14;17(21):21912-21922. doi: 10.1021/acsnano.3c08244. Epub 2023 Oct 18. ACS Nano. 2023. PMID: 37851525 Free PMC article.
References
-
- Seeman N. C. Sleiman H. F. Nat. Rev. Mater. 2017;3:17068.
-
- Yang D. Hartman M. R. Derrien T. L. Hamada S. An D. Yancey K. G. Cheng R. Ma M. Luo D. Acc. Chem. Res. 2014;47:1902–1911. - PubMed
-
- Kwak M. Herrmann A. Angew. Chem., Int. Ed. 2010;49:8574–8587. - PubMed
-
- Jia F. Li H. Chen R. Zhang K. Bioconjugate Chem. 2019;30:1880–1888. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

