Continuous Low-Dose Apatinib Combined With WBRT Significantly Reduces Peritumoral Edema and Enhances the Efficacy of Symptomatic Multiple Brain Metastases in NSCLC
- PMID: 33955301
- PMCID: PMC8111549
- DOI: 10.1177/15330338211011968
Continuous Low-Dose Apatinib Combined With WBRT Significantly Reduces Peritumoral Edema and Enhances the Efficacy of Symptomatic Multiple Brain Metastases in NSCLC
Abstract
Background: Symptomatic multiple brain metastases with peritumoral brain edema (PTBE) occur in non-small cell lung cancer patients (NSCLC) who are without driver mutations or are resistant to epidermal growth factor tyrosine kinase (EGFR-TKI) are often associated with an unfavorable prognosis. Whole brain radiation therapy (WBRT) which comes with many complications and unsatisfactory effects, is the only option for the treatment. Previous studies have shown that bevacizumab can reduce the volume of PTBE and improve efficiency of radiotherapy. This study evaluated the effects and safety of apatinib combined with WBRT in NSCLC patients with symptomatic multiple brain metastases and PTBE.
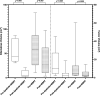
Methods: We performed a retrospective review of 34 patients with symptomatic multiple brain metastases from NSCLC (number >4, and at least 1 measurable brain metastasis lesion with cerebral edema). Intracranial objective response rate (IORR), peritumoral edema and intracranial tumor volumetric measurement, Karnofsky performance status (KPS) and adverse events (AEs) were evaluated. Median intracranial progression-free survival (mIPFS) and median overall survival (mOS) were also analyzed.
Results: Thirteen cases received apatinib (125 mg or 250 mg, QD, oral) combined with WBRT and 21 cases received chemotherapy combined with WBRT were inclued. Apatinib combination group can better reduce the volume of intracranial tumors and PTBE and total steroid dosage used. It was associated with a better IORR (84.6% vs 47.6%, P = 0.067), longer mIPFS (6.97 vs 4.77months; P = 0.014). There was no significant difference in mOS(7.70 vs 6.67 months; P = 0.14) between the 2 groups. The most common adverse events of apatinib combination WBRT included grade 1/2 nausea (4/13), fatigue (3/13), hypertension (2/13) and white blood cell decrease (2/13). No grade 3/4 AEs were observed.
Conclusion: Apatinib plus WBRT is well tolerated and may be a potential choice for relapsed or drug-resistant advanced NSCLC patients with symptomatic multiple brain metastases and PTBE.
Keywords: apatinib; brain metastases; non-small cell lung cancer; peritumoral brain edema; radiation therapy; whole brain radiotherapy.
Conflict of interest statement
Figures



References
-
- Varlotto JM, Flickinger JC, Niranjan A, Bhatnagar AK, Kondziolka D, Lunsford LD. Analysis of tumor control and toxicity in patients who have survived at least one year after radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2003;57(2):452–464. doi:10.1016/S0360-3016(03)00568-6 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

