Rac-maninoff and Rho-vel: The symphony of Rho-GTPase signaling at excitatory synapses
- PMID: 33955328
- PMCID: PMC9707551
- DOI: 10.1080/21541248.2021.1885264
Rac-maninoff and Rho-vel: The symphony of Rho-GTPase signaling at excitatory synapses
Abstract
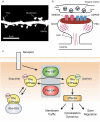
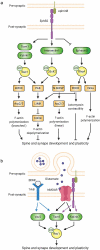
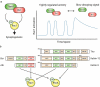
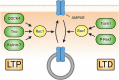
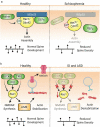
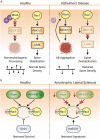
Synaptic connections between neurons are essential for every facet of human cognition and are thus regulated with extreme precision. Rho-family GTPases, molecular switches that cycle between an active GTP-bound state and an inactive GDP-bound state, comprise a critical feature of synaptic regulation. Rho-GTPases are exquisitely controlled by an extensive suite of activators (GEFs) and inhibitors (GAPs and GDIs) and interact with many different signalling pathways to fulfill their roles in orchestrating the development, maintenance, and plasticity of excitatory synapses of the central nervous system. Among the mechanisms that control Rho-GTPase activity and signalling are cell surface receptors, GEF/GAP complexes that tightly regulate single Rho-GTPase dynamics, GEF/GAP and GEF/GEF functional complexes that coordinate multiple Rho-family GTPase activities, effector positive feedback loops, and mutual antagonism of opposing Rho-GTPase pathways. These complex regulatory mechanisms are employed by the cells of the nervous system in almost every step of development, and prominently figure into the processes of synaptic plasticity that underlie learning and memory. Finally, misregulation of Rho-GTPases plays critical roles in responses to neuronal injury, such as traumatic brain injury and neuropathic pain, and in neurodevelopmental and neurodegenerative disorders, including intellectual disability, autism spectrum disorder, schizophrenia, and Alzheimer's Disease. Thus, decoding the mechanisms of Rho-GTPase regulation and function at excitatory synapses has great potential for combatting many of the biggest current challenges in mental health.
Keywords: AMPA receptor; Cdc42; LTD; LTP; NMDA receptor; Rac1; RhoA; dendritic spine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- Markus EJ, Petit TL, LeBoutillier JC.. Synaptic structural changes during development and aging. Developmental Brain Research. 1987;35(2):239–248. - PubMed
-
- Freeman AR. Polyfunctional role of glutamic acid in excitatory synaptic transmission. Prog Neurobiol. 1976;6(2):137–153. - PubMed
-
- Jahr CE, Lester RA. Synaptic excitation mediated by glutamate-gated ion channels. Curr Opin Neurobiol. 1992;2(3):270–274. - PubMed
-
- Mody I, De Koninck Y, Otis TS, et al. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17(12):517–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
