Pyropia yezoensis protein protects against TNF‑α‑induced myotube atrophy in C2C12 myotubes via the NF‑κB signaling pathway
- PMID: 33955507
- PMCID: PMC8127067
- DOI: 10.3892/mmr.2021.12125
Pyropia yezoensis protein protects against TNF‑α‑induced myotube atrophy in C2C12 myotubes via the NF‑κB signaling pathway
Abstract
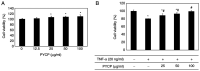
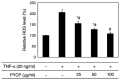
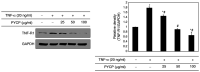
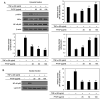
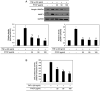
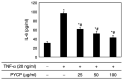
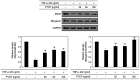
The protein extracted from red algae Pyropia yezoensis has various biological activities, including anti‑inflammatory, anticancer, antioxidant, and antiobesity properties. However, the effects of P. yezoensis protein (PYCP) on tumor necrosis factor‑α (TNF‑α)‑induced muscle atrophy are unknown. Therefore, the present study investigated the protective effects and related mechanisms of PYCP against TNF‑α‑induced myotube atrophy in C2C12 myotubes. Treatment with TNF‑α (20 ng/ml) for 48 h significantly reduced myotube viability and diameter and increased intracellular reactive oxygen species levels; these effects were significantly reversed in a dose‑dependent manner following treatment with 25‑100 µg/ml PYCP. PYCP inhibited the expression of TNF receptor‑1 in TNF‑α‑induced myotubes. In addition, PYCP markedly downregulated the nuclear translocation of nuclear factor‑κB (NF‑κB) by inhibiting the phosphorylation of inhibitor of κB. Furthermore, PYCP treatment suppressed 20S proteasome activity, IL‑6 production, and the expression of the E3 ubiquitin ligases, atrogin‑1/muscle atrophy F‑box and muscle RING‑finger protein‑1. Finally, PYCP treatment increased the protein expression levels of myoblast determination protein 1 and myogenin in TNF‑α‑induced myotubes. The present findings indicate that PYCP may protect against TNF‑α‑induced myotube atrophy by inhibiting the proinflammatory NF‑κB pathway.
Keywords: Pyropia yezoensis protein; aging; myotube atrophy; sarcopenia; tumor necrosis factor‑α.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Pyropia yezoensis peptide PYP1‑5 protects against dexamethasone‑induced muscle atrophy through the downregulation of atrogin1/MAFbx and MuRF1 in mouse C2C12 myotubes.Mol Med Rep. 2017 Jun;15(6):3507-3514. doi: 10.3892/mmr.2017.6443. Epub 2017 Apr 7. Mol Med Rep. 2017. PMID: 28393223 Free PMC article.
-
Pyropia yezoensis Protein Prevents Dexamethasone-Induced Myotube Atrophy in C2C12 Myotubes.Mar Drugs. 2018 Dec 8;16(12):497. doi: 10.3390/md16120497. Mar Drugs. 2018. PMID: 30544821 Free PMC article.
-
Protective Effect of Pyropia yezoensis Peptide on Dexamethasone-Induced Myotube Atrophy in C2C12 Myotubes.Mar Drugs. 2019 May 11;17(5):284. doi: 10.3390/md17050284. Mar Drugs. 2019. PMID: 31083497 Free PMC article.
-
Forkhead Box O Signaling Pathway in Skeletal Muscle Atrophy.Am J Pathol. 2022 Dec;192(12):1648-1657. doi: 10.1016/j.ajpath.2022.09.003. Epub 2022 Sep 27. Am J Pathol. 2022. PMID: 36174679 Review.
-
Emerging Strategies Targeting Catabolic Muscle Stress Relief.Int J Mol Sci. 2020 Jun 30;21(13):4681. doi: 10.3390/ijms21134681. Int J Mol Sci. 2020. PMID: 32630118 Free PMC article. Review.
Cited by
-
A Comprehensive Review of Pathological Mechanisms and Natural Dietary Ingredients for the Management and Prevention of Sarcopenia.Nutrients. 2023 Jun 3;15(11):2625. doi: 10.3390/nu15112625. Nutrients. 2023. PMID: 37299588 Free PMC article. Review.
-
Development and Verification of a Combined Diagnostic Model for Sarcopenia with Random Forest and Artificial Neural Network.Comput Math Methods Med. 2022 Aug 23;2022:2957731. doi: 10.1155/2022/2957731. eCollection 2022. Comput Math Methods Med. 2022. PMID: 36050999 Free PMC article.
-
Characterization of Undiscovered miRNA Involved in Tumor Necrosis Factor Alpha-Induced Atrophy in Mouse Skeletal Muscle Cell Line.Int J Mol Sci. 2024 May 31;25(11):6064. doi: 10.3390/ijms25116064. Int J Mol Sci. 2024. PMID: 38892252 Free PMC article.
-
Inflammation: Roles in Skeletal Muscle Atrophy.Antioxidants (Basel). 2022 Aug 29;11(9):1686. doi: 10.3390/antiox11091686. Antioxidants (Basel). 2022. PMID: 36139760 Free PMC article. Review.
-
Fermented Oyster Extract Attenuated Dexamethasone-Induced Muscle Atrophy by Decreasing Oxidative Stress.Molecules. 2021 Nov 25;26(23):7128. doi: 10.3390/molecules26237128. Molecules. 2021. PMID: 34885708 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

