One-Step Synthesis of a Durable and Liquid-Repellent Poly(dimethylsiloxane) Coating
- PMID: 33955585
- PMCID: PMC11468872
- DOI: 10.1002/adma.202100237
One-Step Synthesis of a Durable and Liquid-Repellent Poly(dimethylsiloxane) Coating
Abstract
Coatings with low sliding angles for liquid drops have a broad range of applications. However, it remains a challenge to have a fast, easy, and universal preparation method for coatings that are long-term stable, robust, and environmentally friendly. Here, a one-step grafting-from approach is reported for poly(dimethylsiloxane) (PDMS) brushes on surfaces through spontaneous polymerization of dichlorodimethylsilane fulfilling all these requirements. Drops of a variety of liquids slide off at tilt angles below 5°. This non-stick coating with autophobicity can reduce the waste of water and solvents in cleaning. The strong covalent attachment of the PDMS brush to the substrate makes them mechanically robust and UV-tolerant. Their resistance to high temperatures and to droplet sliding erosion, combined with the low film thickness (≈8 nm) makes them ideal candidates to solve the long-term degradation issues of coatings for heat-transfer surfaces.
Keywords: adhesion; liquid-repellency; poly(dimethylsiloxane); polymer brushes; wetting.
© 2021 The Authors. Advanced Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


 and ∆Θ:
and ∆Θ:  ) and n‐hexadecane (Θ
ACA:
) and n‐hexadecane (Θ
ACA:  and ∆Θ:
and ∆Θ:  ) repellency of the PDMS brushes versus sonication‐washing time. e) Water (Θ
ACA:
) repellency of the PDMS brushes versus sonication‐washing time. e) Water (Θ
ACA:  and ∆Θ:
and ∆Θ:  ) and n‐hexadecane (Θ
ACA:
) and n‐hexadecane (Θ
ACA:  and ∆Θ:
and ∆Θ:  ) repellency of the PDMS brushes resisting tape‐peeling tests.
) repellency of the PDMS brushes resisting tape‐peeling tests.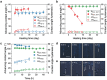

References
-
- Park K.‐C., Kim P., Grinthal A., He N., Fox D., Weaver J. C., Aizenberg J., Nature 2016, 531, 78. - PubMed
-
- a) Cho H. J., Preston D. J., Zhu Y., Wang E. N., Nat. Rev. Mater. 2016, 2, 16092;
- b) Daniel S., Chaudhury M. K., Chen J. C., Science 2001, 291, 633. - PubMed
-
- Wang X., Wang Z., Heng L., Jiang L., Adv. Funct. Mater. 2019, 30, 1902686.
-
- a) Liu T. L., Kim C.‐J. C., Science 2014, 346, 1096; - PubMed
- b) Deng X., Mammen L., Butt H.‐J., Vollmer D., Science 2012, 335, 67; - PubMed
- c) Tuteja A., Choi W., Ma M., Mabry J. M., Mazzella S. A., Rutledge G. C., McKinley G. H., Cohen R. E., Science 2007, 318, 1618; - PubMed
- d) Pan S., Guo R., Björnmalm M., Richardson J. J., Li L., Peng C., Bertleff‐Zieschang N., Xu W., Jiang J., Caruso F., Nat. Mater. 2018, 17, 1040. - PubMed
-
- Zushi Y., Hogarh J. N., Masunaga S., Clean Technol. Environ. 2012, 14, 9.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

