Motorized Macrocycle: A Photo-responsive Host with Switchable and Stereoselective Guest Recognition
- PMID: 33955650
- PMCID: PMC8361693
- DOI: 10.1002/anie.202104285
Motorized Macrocycle: A Photo-responsive Host with Switchable and Stereoselective Guest Recognition
Abstract
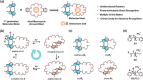
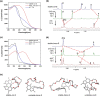
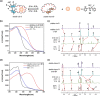
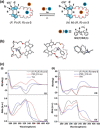
Designing photo-responsive host-guest systems can provide versatile supramolecular tools for constructing smart systems and materials. We designed photo-responsive macrocyclic hosts, modulated by light-driven molecular rotary motors enabling switchable chiral guest recognition. The intramolecular cyclization of the two arms of a first-generation molecular motor with flexible oligoethylene glycol chains of different lengths resulted in crown-ether-like macrocycles with intrinsic motor function. The octaethylene glycol linkage enables the successful unidirectional rotation of molecular motors, simultaneously allowing the 1:1 host-guest interaction with ammonium salt guests. The binding affinity and stereoselectivity of the motorized macrocycle can be reversibly modulated, owing to the multi-state light-driven switching of geometry and helicity of the molecular motors. This approach provides an attractive strategy to construct stimuli-responsive host-guest systems and dynamic materials.
Keywords: capture and release; host-guest interactions; motorized macrocycles; photo-responsiveness; stereoselectivity.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- None
-
- Cram D. J., Angew. Chem. Int. Ed. Engl. 1988, 27, 1009–1020;
- Angew. Chem. 1988, 100, 1041–1052;
-
- Lehn J.-M., Angew. Chem. Int. Ed. Engl. 1988, 27, 89–112;
- Angew. Chem. 1988, 100, 91–116;
-
- Pedersen C. J., Angew. Chem. Int. Ed. Engl. 1988, 27, 1021–1027;
- Angew. Chem. 1988, 100, 1053–1059.
-
- None
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

