Association Between Vaccination With BNT162b2 and Incidence of Symptomatic and Asymptomatic SARS-CoV-2 Infections Among Health Care Workers
- PMID: 33956048
- PMCID: PMC8220476
- DOI: 10.1001/jama.2021.7152
Association Between Vaccination With BNT162b2 and Incidence of Symptomatic and Asymptomatic SARS-CoV-2 Infections Among Health Care Workers
Abstract
Importance: Randomized clinical trials have provided estimates of the effectiveness of the BNT162b2 vaccine against symptomatic SARS-CoV-2 infection, but its effect on asymptomatic infections remains unclear.
Objective: To estimate the association of vaccination with the Pfizer-BioNTech BNT162b2 vaccine with symptomatic and asymptomatic SARS-CoV-2 infections among health care workers.
Design, setting, and participants: This was a single-center, retrospective cohort study conducted at a tertiary medical center in Tel Aviv, Israel. Data were collected on symptomatic and asymptomatic SARS-CoV-2 infections confirmed via polymerase chain reaction (PCR) tests in health care workers undergoing regular screening with nasopharyngeal swabs between December 20, 2020, and February 25, 2021. Logistic regression was used to calculate incidence rate ratios (IRRs) comparing the incidence of infection between fully vaccinated and unvaccinated participants, controlling for demographics and the number of PCR tests performed.
Exposures: Vaccination with the BNT162b2 vaccine vs unvaccinated status was ascertained from the employee health database. Full vaccination was defined as more than 7 days after receipt of the second vaccine dose.
Main outcomes and measures: The primary outcome was the regression-adjusted IRR for symptomatic and asymptomatic SARS-CoV-2 infection of fully vaccinated vs unvaccinated health care workers. The secondary outcomes included IRRs for partially vaccinated health care workers (days 7-28 after first dose) and for those considered as late fully vaccinated (>21 days after second dose).
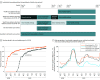
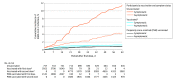
Results: A total of 6710 health care workers (mean [SD] age, 44.3 [12.5] years; 4465 [66.5%] women) were followed up for a median period of 63 days; 5953 health care workers (88.7%) received at least 1 dose of the BNT162b2 vaccine, 5517 (82.2%) received 2 doses, and 757 (11.3%) were not vaccinated. Vaccination was associated with older age compared with those who were not vaccinated (mean age, 44.8 vs 40.7 years, respectively) and male sex (31.4% vs 17.7%). Symptomatic SARS-CoV-2 infection occurred in 8 fully vaccinated health care workers and 38 unvaccinated health care workers (incidence rate, 4.7 vs 149.8 per 100 000 person-days, respectively, adjusted IRR, 0.03 [95% CI, 0.01-0.06]). Asymptomatic SARS-CoV-2 infection occurred in 19 fully vaccinated health care workers and 17 unvaccinated health care workers (incidence rate, 11.3 vs 67.0 per 100 000 person-days, respectively, adjusted IRR, 0.14 [95% CI, 0.07-0.31]). The results were qualitatively unchanged by the propensity score sensitivity analysis.
Conclusions and relevance: Among health care workers at a single center in Tel Aviv, Israel, receipt of the BNT162b2 vaccine compared with no vaccine was associated with a significantly lower incidence of symptomatic and asymptomatic SARS-CoV-2 infection more than 7 days after the second dose. Findings are limited by the observational design.
Conflict of interest statement
Figures
Comment in
-
Technical note: The calculated real world BNT162b2 vaccine efficacy was 88% when accounting for asymptomatic cases.Hum Vaccin Immunother. 2021 Dec 2;17(12):5133-5134. doi: 10.1080/21645515.2021.1994800. Hum Vaccin Immunother. 2021. PMID: 35213948 Free PMC article.
References
-
- US Food and Drug Agency . Emergency Use Authorization for Pfizer-BioNTech COVID-19 vaccine. Accessed February 25, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise...
-
- Israel Ministry of Health . Israeli COVID-19 dataset. Accessed March 22, 2021. https://data.gov.il/dataset/covid-19
-
- Roser M, Ritchie H, Ortiz-Ospina E, Hasell J. Coronavirus pandemic (COVID-19): our world in data. Accessed February 25, 2021. https://ourworldindata.org/coronavirus
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

