Asymptomatic and Symptomatic SARS-CoV-2 Infections After BNT162b2 Vaccination in a Routinely Screened Workforce
- PMID: 33956050
- PMCID: PMC8220512
- DOI: 10.1001/jama.2021.6564
Asymptomatic and Symptomatic SARS-CoV-2 Infections After BNT162b2 Vaccination in a Routinely Screened Workforce
Abstract
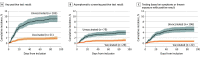
This study aims to describe an association between the Pfizer-BioNTech (BNT162b2) vaccine and decreased risk of symptomatic and asymptomatic infections with SARS-CoV-2 in hospital employees.
Conflict of interest statement
Figures
References
-
- State of Tennessee Health Department. COVID-19 vaccination plan. Posted March 8, 2021. Accessed March 21, 2021. https://www.tn.gov/content/dam/tn/health/documents/cedep/novel-coronavir...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

