Phase 2, randomized, placebo-controlled trial of dapirolizumab pegol in patients with moderate-to-severe active systemic lupus erythematosus
- PMID: 33956056
- PMCID: PMC9194804
- DOI: 10.1093/rheumatology/keab381
Phase 2, randomized, placebo-controlled trial of dapirolizumab pegol in patients with moderate-to-severe active systemic lupus erythematosus
Erratum in
-
Correction to: Phase 2, randomized, placebo-controlled trial of dapirolizumab pegol in patients with moderate-to-severe active systemic lupus erythematosus.Rheumatology (Oxford). 2022 Dec 23;62(1):486. doi: 10.1093/rheumatology/keac326. Rheumatology (Oxford). 2022. PMID: 35700443 Free PMC article. No abstract available.
Abstract
Objective: To evaluate the dose-response, efficacy and safety of dapirolizumab pegol (DZP) in patients with SLE.
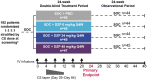
Methods: Adults with moderately to severely active SLE (SLEDAI-2K score ≥6 and ≥1 BILAG A or ≥2 BILAG B domain scores), receiving stable CS (≤40 mg/day prednisone-equivalent), antimalarial or immunosuppressant drugs were included. Patients with stable LN (proteinuria ≤2 g/day) not receiving high-dose CS or CYC were permitted entry. Randomized patients received placebo or i.v. DZP (6/24/45 mg/kg) and standard-of-care (SOC) treatment every 4 weeks to week 24, after which patients received only SOC to week 48. The primary objective was to establish a dose-response relationship based on week 24 BILAG-Based Composite Lupus Assessment (BICLA) responder rates.
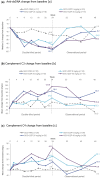
Results: All DZP groups exhibited improvements in clinical and immunological outcomes vs placebo at week 24; however, BICLA responder rates did not fit pre-specified dose-response models [best-fitting model (Emax): P = 0.07]. Incidences of serious treatment-emergent adverse events across DZP groups were low and similar to placebo. Following DZP withdrawal, SLEDAI-2K, physician's global assessment (PGA), BILAG, and Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) scores stabilized; BICLA and SLE Responder Index (SRI-4) responder rates declined (likely due to interventions with disallowed escape medications), BILAG flares increased, and immunologic parameters returned towards baseline.
Conclusions: Although the primary objective was not met, DZP appeared to be well tolerated, and patients exhibited improvements across multiple clinical and immunological measures of disease activity after 24 weeks relative to placebo. The potential clinical benefit of DZP warrants further investigation.
Keywords: CD40 ligand; SLE; dapirolizumab pegol; lupus; systemic lupus erythematosus.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures

References
-
- Rahman A, Isenberg DA.. Systemic lupus erythematosus. N Engl J Med 2008;358:929–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

