Patient, nurse, and physician preferences: final results of the CONVENIENCE study evaluating pegfilgrastim prophylaxis via pre-filled syringe or on-body injector in cancer patients
- PMID: 33956213
- PMCID: PMC8464571
- DOI: 10.1007/s00520-021-06230-9
Patient, nurse, and physician preferences: final results of the CONVENIENCE study evaluating pegfilgrastim prophylaxis via pre-filled syringe or on-body injector in cancer patients
Abstract
Purpose: The on-body injector (OBI) automatically delivers pegfilgrastim the day after chemotherapy (CTx), thus eliminating the need of return visits to the medical office for guideline-compliant pegfilgrastim administration. The CONVENIENCE study aimed to evaluate patient, nurse, and physician preferences as well as health economics for pegfilgrastim administration either with OBI or manually using a pre-filled syringe (PS).
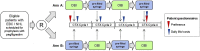
Methods: Patients with early breast cancer, receiving two or three weekly anthracycline/cyclophosphamide or three weekly taxane-based CTx, and patients with Non-Hodgkin lymphoma (NHL) receiving first-line R-CHOP-14 or -21 were randomized 1:1 to receive both pegfilgrastim application forms for four consecutive CTx cycles in an alternating sequence starting either with OBI or PS. Primary endpoint was patient preference, assessed by questionnaires.
Results: A total of 308 patients were evaluable in the per-protocol analysis. Patients slightly preferred OBI over PS (OBI, n = 133, 43.2%; vs. PS, n = 111, 36.0%; p-value = 0.159), while study nurses slightly preferred PS (n = 19, 46.3%) over OBI (n = 18, 43.9%) and physicians clearly preferred PS (n = 24, 58.8%) over OBI (n = 15, 36.6%). Among patients with preference for OBI, saving of time was their major reason for preference (53.4%). Pegfilgrastim was administered 24-72 h after each CTx cycle in 97.6% of OBI and 63.1% of PS applications.
Conclusion: The OBI was slightly preferred by patients and saving time was the major reason for their preference. PS was physicians' most preferable choice and slightly preferred by nurses. Using OBI, pegfilgrastim was almost always administered within the time period recommended by current guidelines, while it was often not applied as specified using PS.
Trial registration: No: ClinicalTrials.gov No. NCT03619993. Registered on June 25, 2018.
Keywords: Manual injection; On-body injector; Patient/physician/nurse preference; Pegfilgrastim.
© 2021. The Author(s).
Conflict of interest statement
D. Semsek, G. Rogmans, U. Hutzschenreuter, T. Fietz, J. Harde, S. Zacharias, A. Lorenz, M.-O. Zahn, D. Guth, S. Liebers, M. Berghorn, S. Grebhardt, C. Matillon, G. Egerer, and K. Potthoff have nothing to disclose.
M. Metz reports personal fees from iOMEDICO, during the conduct of the study; and personal fees from Novartis, BMS, MSD, Amgen, Roche, Biotest, Medac, AbbVie, Celgene, Hexal, Pfizer, Astra Zeneca, Octapharma, Lilly, Sanofi Genzyme, Novartis, Bristol Myers Squibb, and Boehringer Ingelheim, outside the submitted work.
C. Hielscher reports personal fees from Roche, Amgen, Pfizer, and Astra Zeneca and non-financial support from Oncovis, outside the submitted work.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

