Genetic analysis of the Drosophila ESCRT-III complex protein, VPS24, reveals a novel function in lysosome homeostasis
- PMID: 33956855
- PMCID: PMC8101729
- DOI: 10.1371/journal.pone.0251184
Genetic analysis of the Drosophila ESCRT-III complex protein, VPS24, reveals a novel function in lysosome homeostasis
Abstract
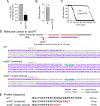
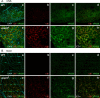
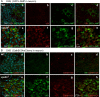
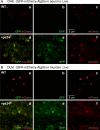
The ESCRT pathway is evolutionarily conserved across eukaryotes and plays key roles in a variety of membrane remodeling processes. A new Drosophila mutant recovered in our forward genetic screens for synaptic transmission mutants mapped to the vps24 gene encoding a subunit of the ESCRT-III complex. Molecular characterization indicated a loss of VPS24 function, however the mutant is viable and thus loss of VPS24 may be studied in a developed multicellular organism. The mutant exhibits deficits in locomotion and lifespan and, notably, these phenotypes are rescued by neuronal expression of wild-type VPS24. At the cellular level, neuronal and muscle cells exhibit marked expansion of a ubiquitin-positive lysosomal compartment, as well as accumulation of autophagic intermediates, and these phenotypes are rescued cell-autonomously. Moreover, VPS24 expression in glia suppressed the mutant phenotype in muscle, indicating a cell-nonautonomous function for VPS24 in protective intercellular signaling. Ultrastructural analysis of neurons and muscle indicated marked accumulation of the lysosomal compartment in the vps24 mutant. In the neuronal cell body, this included characteristic lysosomal structures associated with an expansive membrane compartment with a striking tubular network morphology. These findings further define the in vivo roles of VPS24 and the ESCRT pathway in lysosome homeostasis and their potential contributions to neurodegenerative diseases characterized by defective ESCRT or lysosome function.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

