In-Depth Structural Characterization and Quantification of Cerebrosides and Glycosphingosines with Gas-Phase Ion Chemistry
- PMID: 33957046
- PMCID: PMC8579694
- DOI: 10.1021/acs.analchem.1c01021
In-Depth Structural Characterization and Quantification of Cerebrosides and Glycosphingosines with Gas-Phase Ion Chemistry
Abstract
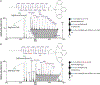
Cerebrosides (n-HexCer) and glycosphingosines (n-HexSph) constitute two sphingolipid subclasses. Both are comprised of a monosaccharide headgroup (glucose or galactose in mammalian cells) linked via either an α- or β-glycosidic linkage to the sphingoid backbone (n = α or β, depending upon the nature of the linkage to the anomeric carbon of the sugar). Cerebrosides have an additional amide-bonded fatty acyl chain linked to the sphingoid backbone. While differentiating the multiple isomers (i.e. glucose vs galactose, α- vs β-linkage) is difficult, it is crucial for understanding their specific biological roles in health and disease states. Shotgun tandem mass spectrometry has been a powerful tool in both lipidomics and glycomics analysis but is often limited in its ability to distinguish isomeric species. This work describes a new strategy combining shotgun tandem mass spectrometry with gas-phase ion chemistry to achieve both differentiation and quantification of isomeric cerebrosides and glycosphingosines. Briefly, deprotonated cerebrosides, [n-HexCer-H]-, or glycosphingosines, [n-HexSph-H]-, are reacted with terpyridine (Terpy) magnesium complex dications, [Mg(Terpy)2]2+, in the gas phase to produce a charge-inverted complex cation, [n-HexCer-H+MgTerpy]+ or [n-HexSph-H+MgTerpy]+. The collision-induced dissociation (CID) of the charge-inverted complex cations leads to significant spectral differences between the two groups of isomers, α-GalCer, β-GlcCer, and β-GalCer for cerebrosides and α-GlcSph, α-GalSph, β-GlcSph, and β-GalSph for glycosphingosines, which allows for isomer distinction. Moreover, we describe a quantification strategy with the normalized percent area extracted from selected diagnostic ions that quantify either three isomeric cerebroside or four isomeric glycosphingosine mixtures. The analytical performance was also evaluated in terms of accuracy, repeatability, and interday precision. Furthermore, CID of the product ions resulting from 443 Da loss from the charge-inverted complex cations ([n-HexCer-H+MgTerpy]+) has been performed and demonstrated for localization of the double-bond position on the amide-bonded monounsaturated fatty acyl chain in the cerebroside structure. The proposed strategy was successfully applied to the analysis of total cerebroside extracts from the porcine brain, providing in-depth structural information on cerebrosides from a biological mixture.
Figures
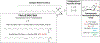
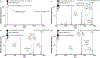

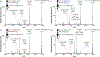
References
-
- Breton C; Šnajdrová L; Jeanneau C; Koča J; Imberty A, Structures and mechanisms of glycosyltransferases. Glycobiology 2005, 16 (2), 29R–37R. - PubMed
-
- Schnaar RL; Kinoshita T, Glycosphingolipids. In Essentials of Glycobiology [Internet], 3rd edition ed.; Varkim A; Cummings RD; Esko JD; Stanley P; Hart GW; Aebi M; Darvill AG; Kinoshita T; Packer NH; Prestegard JH; Schnaar RL; Seeberger PH, Ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press, 2017. - PubMed
-
- Han X; Gross RW, Shotgun lipidomics: Electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev 2005, 24 (3), 367–412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

